Diffuse Nodular Lymphoid Hyperplasia (DNLH) of the Gastrointestinal Tract (GIT) is an uncommon, benign reactive lesion occurring in all age groups. It is seen distributed throughout the GIT. DNLH is characterized by diffuse mucosal polypoid masses of 2-5 mm diameter. Commonly mistaken for various polyposis syndromes on endoscopy, It may present with abdominal pain, recurrent hematochezia, intussusception, intestinal obstruction and anaemia. Childhood DNLH is associated with various etiologies from food hypersensitivity to immunodeficiency syndromes. As DNLH is mostly a benign, reactive process, histopathology plays an important role in its recognition to prevent misdiagnosis and radical procedures.
Here, we present a rare case of DNLH of colon in a two year old female child, presenting with abdominal distension. Colonoscopy revealed multiple colonic polyps. Total colectomy specimen revealed multiple, sessile polyps of 0.2 to 0.3cm carpeting the entire colonic mucosa. Microscopy revealed polyps comprised of lymphoid follicles with germinal centre in the mucosal and submucosal location.
Case Report
A two-year-old female child presented to the outpatient department of paediatric surgery unit of our hospital with history of abdominal pain, abdominal distension and swelling of lower limbs of three months duration. On examination child was pale, had pitting edema of both lower limbs and distension of abdomen with reduced bowel sounds. Per rectal examination revealed persistent rectal prolapse of non-reducible type. Laboratory tests revealed haemoglobin 7.4gm/dl, Packed Cell Volume (PCV) 24.3%, Mean Corpuscular Volume (MCV) 66fl, Mean Corpuscular Haemoglobin (MCH) 19.9pg, Mean Corpuscular Haemoglobin Concentration (MCHC) 30.2%. Peripheral smear showed microcytic hypochromic anaemia. Colonoscopy showed multiple polyps of the colon. Patient underwent total colectomy, endorectal mucosectomy and pull through. Post-operatively patient developed intestinal adhesions and small intestinal intussusception, for which adhesiolysis and reduction of the intussusception was done. Patient was discharged after uneventful post-operative recovery but later lost to follow up.
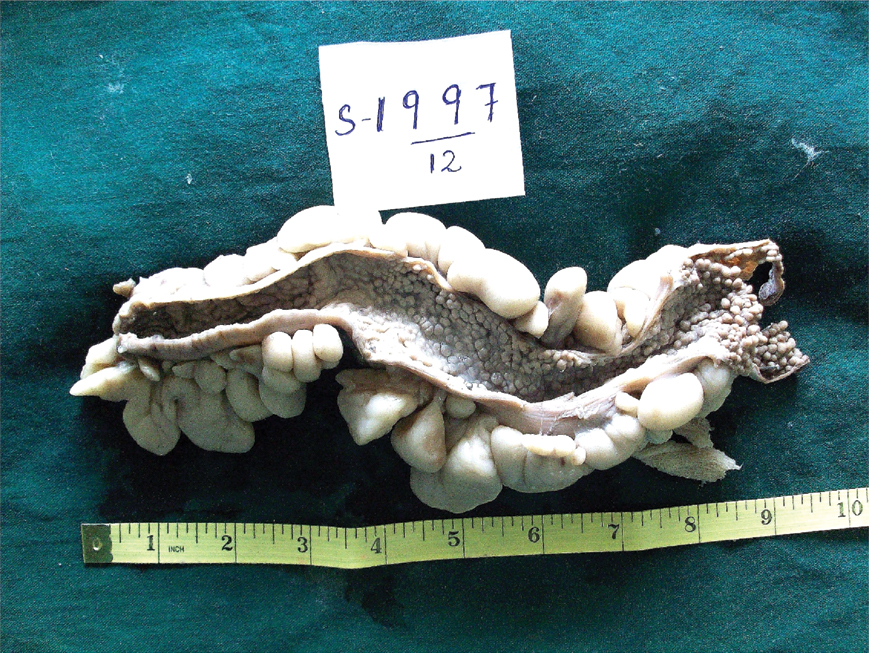
Macroscopic examination of the total colectomy specimen measuring 26 cm in length showed prominent appendices epiploicae and multiple pericolic lymph nodes varying between 0.4 to 0.5 cm in size. The mucosa of the colon was carpeted by multiple, sessile, flesh coloured polyps, measuring 0.2 to 0.3 cm [Table/Fig-1].
Mucosa of the colon studded with innumerable sessile fleshy polyps.
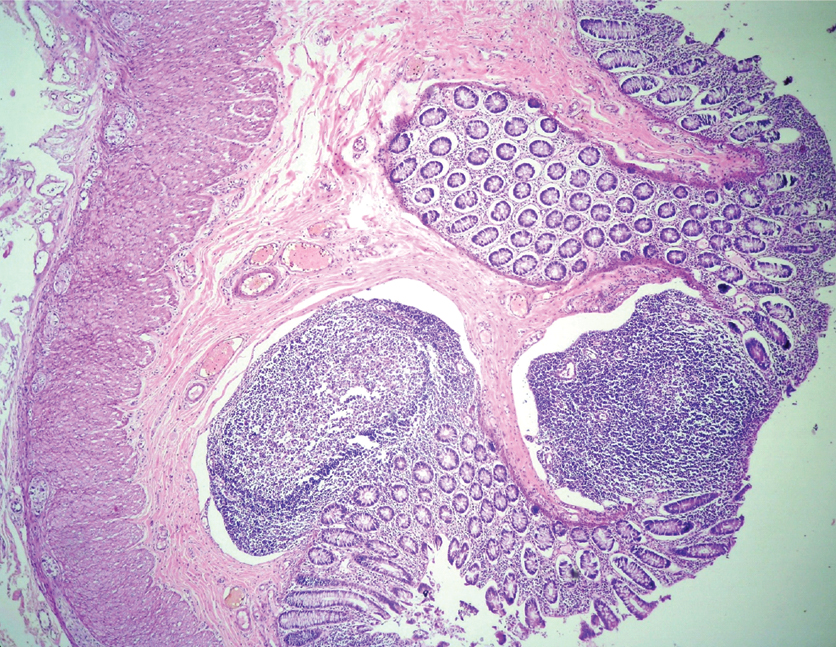
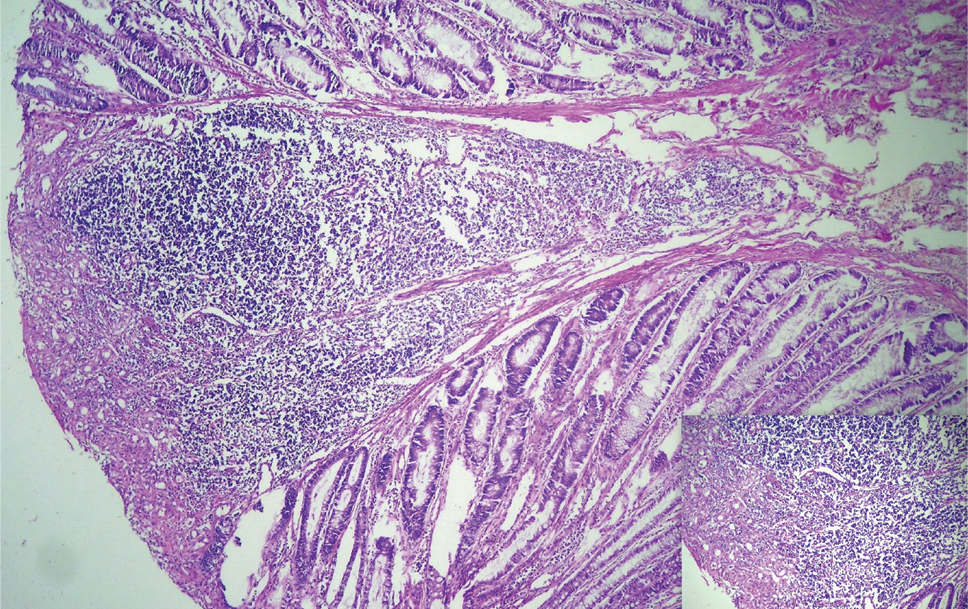
Multiple polyps were subjected to histopathological examination, all of which revealed lymphoid follicles many with germinal centers [Table/Fig-2]. The overlying epithelium showed focal ulceration, features of mucus depletion, increased mitotic figures and regenerative atypia [Table/Fig-3]. Submucosa showed ectatic and congested vascular channels. All the lymph nodes showed follicular hyperplasia. A diagnosis of diffuse nodular lymphoid hyperplasia of colon and reactive lymphoid hyperplasia of pericolic lymph nodes was made.
Photomicrograph showing mucosal and submucosal lymphoid follicles (H&E stain 40X).
Photomicrograph showing mucosal lymphoid aggregation (H&E stain 100X); Inset showing ulceration of the mucosa and granulation tissue response (H&E stain 400X).
Discussion
Nodular Lymphoid Hyperplasia (NLH) of the GIT is a benign, reactive lesion, characterized by multiple small nodules of 2-10 mm diameter. Known by other names such as lymphoid polyposis and pseudo lymphoma, [1] it is an uncommon lesion, occurring in both adults and children, with the youngest being a 10 month infant [2].
Nodular Lymphoid Hyperplasia occurs in diffuse and focal forms across all age groups [3]. Focal lesions are single, variably circumscribed, produce wall thickening and are commonly seen in adults [1,4]. Diffuse form also called as DNLH is common in children under 10 years of age, when general lymphatic hyperplasia is common [2,3]. It may involve variable segments of the small intestine, large intestine or both. Colon AR et al., in their review of 147 children with lymphoid polyposis found DNLH involving the colon in majority (57%), while small bowel was involved in 43% of cases [5].
Diffuse Nodular Lymphoid Hyperplasia can be asymptomatic or symptomatic. When symptomatic they can present with anaemia, abdominal pain, recurrent gastrointestinal bleeding, refractory constipation, chronic diarrhoea, intestinal obstruction or intussusception [1,2,6]. In a large study, involving 65 cases of NLH of terminal ileum, diarrhoea was the most common symptom seen in 70.8%, followed by abdominal pain in 60%, hematochezia in 46.2%, anaemia in 40% and hypoproteinemia in 21.5% of patients [7]. Our case presented with anaemia, abdominal pain, distension of abdomen and rectal prolapse.
Etiopathogenesis of NLH is not known, though many theories are proposed. In childhood DNLH, delayed type of food hypersensitivity has been implicated. Iacono G et al., studied 245 children who underwent colonoscopy for various reasons and found isolated NLH in 21.2% (52/245) of patients. Of these 83% had cow’s milk protein or multiple food hypersensitivity (p<0.0001). Such children when put on oligoantigenic diet reported alleviation of their symptoms, which reappeared following food challenge suggesting that isolated NLH of colon and terminal ileum could be due to food hypersensitivity [8].
Immunodeficiency states like hypogammaglobulinemia, common variable immunodeficiency syndrome, IgA deficiency, Human Immunodeficiency Virus (HIV) infection and coeliac disease have all been associated with NLH, more so in adults [1,6]. Infections due to Giardia lamblia and Helicobacter pylori have also been seen in patients with NLH, albeit in locations other than colon. Repetitive stimulation by an antigenic trigger like infection could lead to hyperplastic lymphoid follicles. Reversal or regression of lymphoid follicles after specific therapy to these infectious agents acts as supportive evidence [9,10]. Viral infections are also implicated in the development of DNLH [1,3].
Sabra A et al., reviewed the brain intestine connection in patients with autistic spectrum disorder. They found Gut Associated Lymphoid Tissue (GALT) involvement due to food allergy in 84%, before the manifestation of the autistic spectrum disorder. They suggested that once the antigen is activated in the Peyer’s patches of the GALT, the antigen, lymphocytes and immunoglobulins continue to search for homing and this could be bronchial, nasal, skin or central nervous system associated lymphoid tissue, the later involved in the autistic spectrum disorder. The association of ileocolonic NLH in children with autistic spectrum disorder could very well explain this [11]. Wakefield AJ et al., also observed ileocolonic NLH in 90% of children with autistic spectrum disorder [12].
Combination of factors could exist sometimes in patients with NLH as described by Hermans PE et al., Hypogammaglobulinemia, infection with Giardia lamblia and NLH of small intestine has been given the term Herman’s syndrome [3,13,14]. Choi JH et al., have reported a case of common variable immunodeficiency syndrome having low levels of serum IgG, IgM, IgA levels, impaired antibody production to hepatitis B vaccination, refractory giardiasis and diffuse nodular lymphoid hyperplasia of small intestine [15]. Our patient was HIV negative with no history of allergy or any behavioural abnormality. Objective assessment for food allergy, immunodeficiency syndromes were not done as the child was lost to follow up.
Colonoscopic findings of DNLH of colon may resemble other polyposis syndromes like familial adenomatous polyposis, juvenile polyposis, hamartomatous polyposis, or hyperplastic polyposis, similar to our case [16]. Barium meal study or air contrast barium enema usually reveals multiple small rounded filling defects in DNLH [2,9]. We did not perform barium studies in our case.
Hence, histopathology determines the exact nature of ileocolonic polyps. Multiple lymphoid follicles with germinal centres both in mucosal and submucosal location gives rise to these nodules/ polyps which sometimes protrude into the lumen causing effacement of overlying villous architecture especially in the ileum [1,9]. When they protrude into the lumen these can act as nidus for prolapse, intussusception or intestinal obstruction, [16] as seen in our case the child had rectal prolapse with features of intestinal obstruction.
The polymorphic nature of these lymphoid follicles without cytologic atypia helps to differentiate these lesions from malignant lymphomas [1]. This distinction becomes important as few cases of DNLH have shown lymphomatous transformation [1,9] and few are associated with intestinal lymphomas. Burkitt’s lymphoma, a rare form of non-Hodgkin’s lymphoma of intestine has been reported in the background of NLH in a patient with selective IgA deficiency [17]. Rarely extra intestinal lymphoma has been reported by Rubio-Tapio A et al., and Jonsson OT et al. [13,18]. Spontaneous remission of DNLH following chemotherapy for associated intestinal and extra intestinal lymphoma and reappearance at relapse has been reported [3,13]. Increased risk of adenomas, gastric and colorectal adenocarcinomas have also been reported [6,16]. Hence, long-term prognosis of patients with DNLH is nebulous, though most authors suggest a benign course especially in children.
Conclusion
Nodular Lymphoid Hyperplasia is an uncommon, benign reactive process with characteristic histopathological findings occurring in both adults and children. It is commonly associated with food hypersensitivity, intestinal infections and immunodeficiency syndromes. Diffuse Nodular Lymphoid Hyperplasia in children appears to have a benign course and in many undergo spontaneous regression. Hence, specific therapy to infection and oligoantigenic diet appears to help these patients, preventing them from undergoing radical procedures like colectomy. Pre-operative Barium meal along with colonoscopy could aid in the diagnosis of DNLH. However, a biopsy with histopathological examination is mandatory to confirm the benign nature.
[1]. Shamina I, Tamanna C, Md. Shahadat H, Mohammed K, Lymphoid polyposis in an adult male- a case reportBSMMU J 2011 4(2):119-121. [Google Scholar]
[2]. William ML, Eldad R, Benign lymphoid hyperplasia of the colonThe West J Med 1977 127(5):416-418. [Google Scholar]
[3]. Andreia A, Nodular lymphoid hyperplasia in the gastrointestinal tract in adult patients: A reviewWorld J Gastrointest Endosc 2014 6(11):534-540. [Google Scholar]
[4]. Ranchod M, Lewin KJ, Dorfman RF, Lymphoid hyperplasia of the gastrointestinal tract: A study of 26 cases and review of the literatureAm J Surg Pathol 1978 2(4):383-400. [Google Scholar]
[5]. Colon AR, DiPalma JS, Leftridge CA, Intestinal lymphonodular hyperplasia of childhood: patterns of presentationJ Clin Gastroenterol 1991 13:163-166. [Google Scholar]
[6]. Piascik M, Rydzewska G, Pawlik M, Milewski J, Furmanek MI, Wronska E, Diffuse nodular lymphoid hyperplasia of the gastrointestinal tract in patient with selective Immunoglobulin A deficiency and sarcoid-like syndrome – case reportAdvances in Medical Sciences 2007 52:296-300. [Google Scholar]
[7]. Lin R, Lu H, Zhou G, Wei Q, Liu Z, Clinicopathological and ileocolonoscopic characteristics in patients with nodular lymphoid hyperplasia in the terminal ileumInt.J.Med.Sci 2017 14(8):750-757. [Google Scholar]
[8]. Iacono G, Ravelli A, Di Prima L, Scalici C, Bolognini S, Chiappa S, Colonic lymphoid nodular Hyperplasia in children: Relationship to food hypersensitivityClin Gastroenterol Hepatol 2007 5(3):361-366. [Google Scholar]
[9]. Ward H, Jalan KN, Maitra TK, Agarwal SK, Mahalanabis D, Small intestinal nodular lymphoid hyperplasia in patients with giardiasis and normal serum immunoglobulinsGut 1983 24(2):120-126. [Google Scholar]
[10]. Mehnaaz SK, Naira SK, Mohammad SK, Diffuse duodenal nodular lymphoid hyperplasia: a large cohort of patients etiologically related to Helicobacter pylori infectionBMC Gastroenterology 2011 11:36 [Google Scholar]
[11]. Sabra A, Corsini L, Tenorio I, Sabra S, Nemer JM, Filho AS, The brain-intestine connection and the autistic spectrum disorderJ Food Allergy 2015 4(1):18-26. [Google Scholar]
[12]. Wakefield AJ, Ashwood P, Limb K, Anthony A, The significance of ileo-colonic lymphoid nodular hyperplasia in children with autistic spectrum disorderEur J Gastroenterol Hepatol 2005 17(8):827-836. [Google Scholar]
[13]. Rubio-Tapia A, Hernandez-Calleros J, Trinidad-Hernandez S, Uscanga L, Clinical characteristics of a group of adults with nodular lymphoid hyperplasia: A single center experienceWorld J Gastroenterol 2006 12(12):1945-1948. [Google Scholar]
[14]. Hermans PE, Diaz-Buxo JA, Stobo JD, Idiopathic late-onset immunoglobulin deficiency. Clinical observations in 50 patientsAm J Med 1976 61:221-237. [Google Scholar]
[15]. Choi JH, Han DS, Kim J, Yi K, Oh Y, Kim Y, Diffuse nodular lymphoid hyperplasia of the intestine caused by Common variable Immunodeficiency and Refractory GiardiasisIntern Med 2017 56(3):283-287. [Google Scholar]
[16]. Eren E, Haldun G, Nevzat SU, Recep A, A case of diffuse nodular lymphoid hyperplasiaTurk J Gastroenterol 2008 19(4):268-270. [Google Scholar]
[17]. Hanich T, Majnaric L, Jankovic D, Sabanovic S, Vcev A, Nodular lymphoid hyperplasia complicated with ileal Burkitt’s lymphoma in an adult patient with selective IgA deficiencyInt Surg Case Rep 2016 30:69-72. [Google Scholar]
[18]. Jonsson OT, Birgisson S, Reykdal S, Resolution of nodular lymphoid hyperplasia of the gastrointestinal tract following chemotherapy for extraintestinal lymphomaDig Dis Sci 2002 47:2463-65. [Google Scholar]