Thyroid malignancies constitute 1% of all malignancies in developed countries. Their incidence is increasing recently and this could be due to advances in radiological and histopathological techniques. Papillary Microcarcinoma Thyroid (PMCT) is the fastest growing type of thyroid cancer [1].
Papillary microcarcinoma is a type of thyroid cancer defined by the World Health Organization (WHO) Histological classification of tumours by two criteria: (i) one cm or smaller in size and (ii) an incidental finding on histopathological examination after lobectomy or total thyroidectomy performed for a larger adenoma or nodular hyperplasia [2].
In surgical series, PMCT is reported in 5–17% of patients who have a thyroidectomy for benign lesions. PMCTs are found in normal thyroids or in multinodular goiters and they account for nearly 50% of new cases of PTC [3].
PMCTs are detected as incidental findings on neck imaging for indications other than thyroid cancer. Unsuspected PMCTs also appear in surgical specimens from operations for benign thyroid disease or parathyroid adenoma. Incidental PMCT has outstanding good prognosis and there is nearly no risk of recurrence or death from PMCT. However, PMCT may be associated with lymph node metastases at presentation and/or neck locoregional recurrences during follow-up [4].
Clinical management of patients with PMCT remains non-standardized. These tumours are generally considered clinically innocuous, although some have an aggressive clinical behaviour [5]. The risk factors associated with aggressive behaviour of PMCT are not well defined.
The need to stratify those relatively few patients with aggressive PMCT from the vast majority who are low-risk is crucial to offer the most appropriate clinical management. The more consistently reported features associated with risk of recurrence or metastasis are tumour multifocality and bilaterality. Other proposed risk factors include younger age, male gender, extensive tumour fibrosis, tumour size, capsular invasion, extrathyroidal extension and mast cell infiltration. This study was attempted to determine the clinical and histomorphological factors that are associated with adverse outcome in patients with PMCT.
Materials and Methods
This was a cohort study done from 2008 to 2015 in the Department of Pathology, Government Medical College, Kozhikode. Study subjects included retrospective cases from 2008 to 2013 and prospective subjects from 2013 to 2015. The patients whose paraffin blocks could not be retrieved were excluded. Informed consent was obtained from the study subjects. Ethical clearance was obtained from the Institutional Ethics Committee.
Clinical details were collected from the patients and case sheets and included age, gender, duration of symptoms and follow-up details for minimum of six months for recurrence. The paraffin embedded blocks and Hematoxylin and Eosin (H&E) stained slides were studied for assessing tumour size, focality, capsular invasion, extrathyroidal extension, histological subtype, thyroiditis, tumour fibrosis, mast cell infiltration and lymph node status.
Tumour fibrosis was graded as follows.
(i) Mild – Mild fibrosis with presence of few inconspicuous, delicate fibrous areas within or at the periphery of tumour nodule.
(ii) Moderate – Moderate fibrosis which is clearly recognizable with multiple fibrotic bands within and at the periphery of tumour.
(iii) Severe – Extensive areas of fibrosis within and at the periphery of the tumour.
Sections from the largest cut surface of thyroid both outside the tumour area and within the tumour area were studied for lymphocytic infiltration. Grading of lymphocytic infiltration was done as follows:
Grade 1: 2-8 foci/4 cm2
Grade 2: 9-40 foci/4 cm2
Grade 3: >40 foci/4 cm2
Grade 4: more than half of glandular parenchyma replaced by lymphocytic infiltrate.
One focus was defined as a collection of more than 50 lymphocytes.
Multifocality was defined as either (i) two or more separate tumours, or (ii) smaller tumour aggregates located close to the main tumour separated by a layer of normal thyroid parenchyma, or (iii) tumour cells within lymphatic channels.
Immunohistochemical staining by CD-117 was done to identify the mast cells and assess the mean mast cell count. The estimation of CD-117 positive mast cells were done by counting them in non-overlapping high power (40X) fields of an Olympus BX43 microscope. The high power field of this microscope has a diameter of 0.55 mm and covers an area of 0.24 mm2. The counts were converted to per unit area (mm2) for purpose of comparison. High tumour mast cell was defined as more than 1 cell/mm2.
Statistical Analysis
The data was entered in the spreadsheets of Microsoft Office Excel and the variables were analyzed using standard analytic techniques with SPSS version 16.0 for Windows. The quantitative variables were expressed as mean and qualitative variables were expressed as percentages. The association between variables were analyzed using Fisher’s exact test as the sample size was small. The p-values were calculated and values<0.05 were taken as statistically significant.
Results
In this study, a total of 55 patients were included. The age group of patients in the study ranged from 24 to 70 years, the mean being 40 years (SD-9.95). The maximum subjects belonged to the age group of 41 to 50 years. Of the 55 subjects, 44 (80%) were females and 11 (20%) were males.
The mean size of the tumour ranged from 0.1 cm to 1 cm (mean- 0.56 cm). The subjects were classified on the basis of tumour size into two groups (≤ 0.5 cm and >0.5 cm). Thirty one of 55 subjects had tumour of size less than 0.5 cm, while 24 had tumour size >0.5 cm.
The subjects were also assessed for capsular invasion, focality of the lesion and severity of tumour fibrosis. The results are shown in [Table/Fig-1].
Comparison of focality, capsular invasion and severity of tumour fibrosis in the sample.
| Parameter | Frequency | Percent (%) |
|---|
| Focality | Unifocal | 51 | 92.7 |
| Multifocal | 4 | 7.3 |
| Capsular invasion | Present | 4 | 7.3 |
| Absent | 51 | 92.7 |
| Severity of tumour fibrosis | Mild | 36 | 65.5 |
| Moderate | 19 | 34.5 |
The background thyroid gland was assessed and the results are shown in [Table/Fig-2]. Six subjects had grade three lymphocytic thyroiditis as shown in [Table/Fig-3].
Assessment of adjacent thyroid in the sample.
| Surrounding thyroid tissue | Frequency | Percent (%) |
|---|
| Nodular colloid goitre | 13 | 23.6 |
| Lymphocyticthyroiditis | Grade 1 | 17 | 30.9 |
| Grade 2 | 15 | 27.3 |
| Grade 3 | 6 | 10.9 |
| Normal | 3 | 5.5 |
| Tuberculous | 1 | 1.8 |
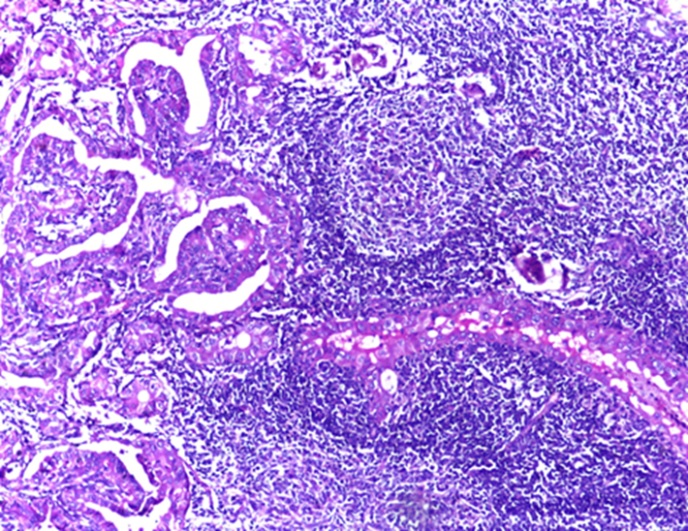
Grade 3 lymphocytic thyroiditis (H&E 40X).
Mast cell infiltration within and near the tumour were assessed in 38 subjects only due to non-availability of good quality blocks for performing immunohistochemical studies. CD 117 staining was done to identify the mast cells. The mean number of mast cells per mm2 was assessed. Ten subjects had high mean mast cell counts (>1/mm2). None of the subjects had extrathyroidal extension. The tumours were also examined to assess the histological subtype of PMCT. Fifty four subjects had the classical variant of PMCT, and one had follicular variant.
The subjects were followed up, with a minimum of six months and maximum of 84 months (seven years); the mean being 3.59 years (SD-2.2).
Of the 55 subjects, five had lymph node metastasis at the time of presentation. However, none developed recurrence or tumour associated mortality during the follow-up period. Lymph node metastasis was taken as the adverse outcome and the subjects were divided into two groups on this basis. Histomorphological and clinical parameters were compared in the 2 groups to determine statisticaly significance of the variables. The results are given in [Table/Fig-4].
Comparison of clinical and histological parameters with occurrence of adverse outcome.
| Parameter | Lymph node present | Lymph node absent | p-value |
|---|
| Age | ≤ 40 years | 2 | 28 | 0.65 |
| >40 years | 3 | 22 |
| Gender | Male | 3 | 8 | 0.049 |
| Female | 2 | 42 |
| Tumour size | ≤ 0.5 cm | 1 | 30 | 0.156 |
| >0.5 cm | 4 | 20 |
| Focality | Unifocal | 3 | 48 | 0.037 |
| Multifocal | 2 | 2 |
| Capsular invasion | present | 2 | 2 | 0.037 |
| absent | 3 | 48 |
| Tumour fibrosis | mild | 1 | 35 | 0.043 |
| moderate | 4 | 15 |
| Lymphocytic thyroiditis | Present | 5 | 33 | 0.309 |
| Absent | 0 | 17 |
| Histological subtype | Classical | 5 | 49 | 1 |
| Follicular | 0 | 1 |
| Mean mast cell count(per mm2) | ≤ 1/mm2 | 1 | 27 | 0.164 |
| >1/ mm2 | 2 | 8 |
* p-values - calculated by Fisher’s exact test.
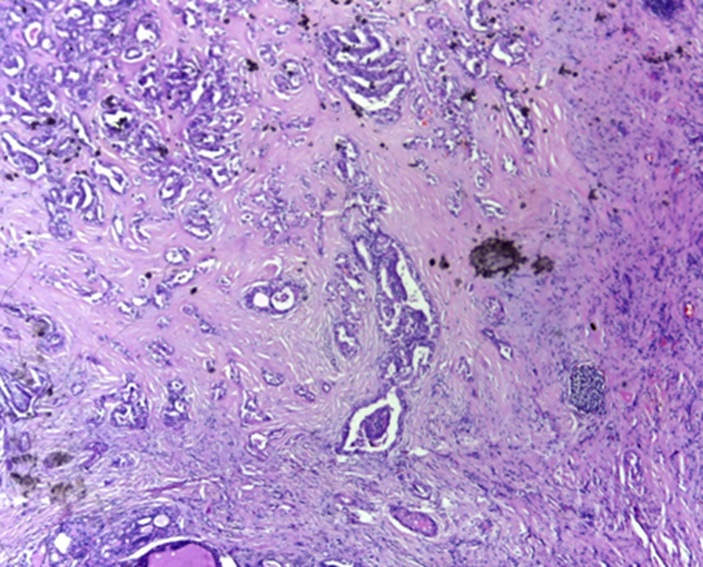
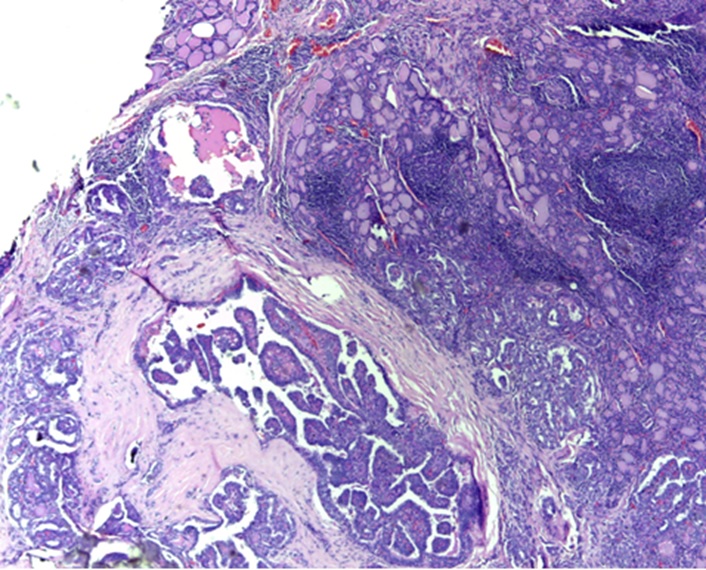
Male gender (p=0.049), multifocality (p=0.037), moderate-severe tumour fibrosis (p=0.043) [Table/Fig-5] showing moderate tumour fibrosis and capsular invasion (p=0.043). [Table/Fig-6] showing capsular invasion by papillary microcarcinoma were found to be significantly associated with lymph node metastasis.
Moderate tumour fibrosis (H&E 4X).
Microscopy Capsular invasion by papillary microcarcinoma (H&E 4X).
Discussion
The incidence of thyroid cancers especially PMCT is increasing in the western world over many years. The relative rate of microcarcinoma has increased because of the improvements in the diagnostic radiology as well as the accurate pathological examination of thin slices of specimens [1].
The age group of patients in the study ranged from 24 to 70 years, the mean being 40 years. This is in concordance to many other studies which reports mean age of diagnosis as 45 to 50 years [6,7].
Striking female predisposition was found in the distribution of the tumour. This was found to be similar to combined results of different studies where males were found to be 17% and females constituting 83% [5-7]. The size of the tumour ranged from 0.1 to 1 cm with a mean of 0.56 cm. Majority of the tumours (56.4%) were 0.5 cm or less in size. However, in similar studies in the west, majority of tumours (76.4%) reported were larger than 0.5 cm [8,9].
Of the 55 subjects, 51 (92.7%) had unifocal lesions; and multifocal lesions were found only in four subjects. Larger case series have demonstrated greater degree of multifocality (36.1%) [10] in papillary microcarcinoma.
Capsular invasion by the tumour was seen in 4 (7.3%) subjects. However, in study by Corapcioglu D et al., [11], capsular invasion was observed in 26.8 % of the subjects.
None of the subjects had extrathyroidal invasion by the lesion. Higher rates of extrathyroidal extension (20%) was noted by Pellegriti G et al., [12].
Thirty six out of 55 subjects had mild fibrosis within and adjacent to the tumour. Ninety subjects had moderate fibrosis. Extensive tumour fibrosis was not seen in any subjects. In studies by Niemeier LA et al., [13], moderate fibrosis was seen in 61% of the subjects.
Of the 55 subjects, 38 (69.1%) had chronic lymphocytic thyroiditis in the adjacent thyroid. This includes six subjects with Hashimoto thyroiditis. This is in concordance to the observation in many studies that chronic lymphocytic thyroiditis exists with papillary carcinoma thyroid and many other neoplasms [14,15].
Adverse outcome was evaluated as presence of lymph node metastasis at the time of presentation or history of recurrence during the follow-up period. 5 (9.09%) out of 55 patients had associated lymph node metastasis at the time of presentation. None of the patients had recurrence or tumour associated mortality during the follow-up period. Lymph node metastasis at the time of presentation strongly correlates with chances of tumour recurrences, and so, it can be reliably used as an indicator of aggressiveness of papillary microcarcinoma, and hence, with the associated adverse outcomes.
Lymph node metastasis was found to be higher in older subjects (>40 years of age), with highest incidence between 41-50 years. However, similar to many other studies, no significant differences was found between these two groups in this study [16].
Of the 11 subjects, 3 (27.3%) male subjects developed lymph node metastasis but only 2 (4.5%) out of 44 females had metastasis. So, although the incidence of papillary microcarcinoma is less in males, there is a significant difference in the outcome in both groups. This coincides with previous studies in identifying gender as an important variable in risk stratification of the patients [17].
Four out of five subjects who had lymph node metastasis had tumours with size greater than 0.5 cm. So, emphasis should be given to tumour size, although no statistical significance could be derived. Studies by Kasai and Sakamoto have reported more frequent lymph node metastasis in tumours >0.5 cm [18].
Of the four subjects, Two (50%) subjects with multifocal lesions had lymph node metastasis. This points towards its role as indicator of prognostication in papillary microcarcinoma. This was also observed in similar studies done by Chow SW et al., [19].
Of the four subjects, Two (50%) subjects with capsular invasion had lymph node metastasis. Hence, this can also be considered as a prognostic factor.
Moderate tumour fibrosis was seen in four (80%) out of five subjects with lymph node metastasis. This was also observed in studies done by Baudin E et al., [20]. The fibrosis can be correlated with tumour desmoplastic response and indicates tumour invasiveness. So, moderate to extensive fibrosis can be considered as an indirect measure of aggressiveness of the tumour. None of the subjects had extrathyroidal extension by the tumour. One subject had follicular variant of papillary microcarcinoma, but was not found to be of any prognostic significance.
All the five subjects with lymph node metastasis had lymphocytic thyroiditis in the surrounding thyroid tissue but this is not found to be significant in prognostication. Some studies have reported that papillary carcinoma with lymphocytic thyroiditis is associated with lower recurrence rate, less aggressive character and better prognosis. However, Kebebew E et al., has reported that lymphocytic thyroiditis is not an independent prognostic three factor which could affect the recurrence rate and distant metastasis [21].
Mast cell infiltration within the tumour was assessed in 38 random subjects which included 35 subjects without lymph node metastasis and four with lymph node metastasis. Two (50%) out of four subjects with lymph node metastasis had high mast cell infiltration (>1/mm2), as compared to eight (22.8%) out of 35 subjects without lymph node metastasis. However, no significant difference can be seen in the two categories.
The assessment of high risk factors in PMCT has important therapeutic implications. The PMCT without risk factors need to be treated with total thyroidectomy and dissection of any palpable cervical lymph node. However, those with high risk nature need to be considered like PTC and require total thyroidectomy followed by radio-iodine ablation. These patients should also be followed up more rigorously for development of recurrences and distant metastases.
Limitation
This study has limitations owing to small sample size and shorter follow-up period. The aggressiveness and adverse outcome of papillary microcarcinoma were ascertained on the basis of lymph node metastasis at the time of presentation. Tumour recurrences were not observed in any of the subjects. Tumour associated mortality is extremely rare in papillary microcarcinoma and is also not observed in the study.
Conclusion
Papillary carcinoma is generally an indolent tumour, however, high risk groups should be recognized. High-risk groups have more propensity for extrathyroidal extension and recurrences. So, more aggressive treatment with radioablation is needed. In this study, male gender, multifocality, capsular invasion and moderate tumour fibrosis were found to be associated with adverse outcome. No statistical significance was found for age, tumour size, histological subtype, thyroiditis and mast cell infiltration. The significance of these variables and their role in prognostication could be elucidated if the cohort of people could be followed-up for more years.
* p-values - calculated by Fisher’s exact test.