Anaemia is a common condition, particularly in young women and in the geriatric population. It is regarded as a significant public health problem in developing countries. Anaemia is defined by the World Health Organisation (WHO) as Haemoglobin (Hb) <120 gm/L in women and Hb <130 gm/L in men. This definition includes the so called pseudo anaemic states (pregnancy, cardiac heart failure and hyperproteinaemia) where, Hb concentration falls as the result of an increase of the plasma volume. Approximately, every fourth person on the earth is anaemic [1].
Megaloblastic anaemia is basically caused by vitamin B12 and/or folic acid deficiency [2]. In megaloblastic anaemia, there is ineffective erythropoiesis in the bone marrow either due to vitamin B12 and/or folic acid deficiency. Vitamin B12 deficiency is mostly related with malabsorption, whereas, folic acid deficiency is frequently caused by low dietary intake [3].
For the diagnosis of megaloblastic anaemia complete blood count, general blood picture and bone marrow aspiration is required. Cytopenias e.g., bicytopenia and pancytopenia are present in the bone marrow. Ineffective erythropoiesis and premature death of cells decreases their output from the bone marrow; hence, anaemia occurs. Megaloblastic anaemia patients bear a risk of developing complications such as congestive heart failure, dementia, enlarged spleen, gallstones, heart attack, impotence, sepsis, shock, sterility and infertility. Vitamin B12 and folic acid levels have been shown to affect haematological parameters too. Individuals with lower vitamin B12 levels have been shown to have significantly lower haemoglobin, haematocrit, mean corpuscular haemoglobin, mean corpuscular haemoglobin concentration and white blood cells including neutrophils. They are said to have higher red cell distribution width and lymphocytes as compared to those having normal vitamin B12 levels [4]. This is proven by the fact that vitamin B12 supplementation in such patients helps in normalisation of these parameters [5]. However, the effect of vitamin B12 and folic acid deficiency does not influence the haematological parameters in similar manner. For low and marginal folic acid deficiency, the Mean Corpuscular Volume (MCV) values have been found to be relatively more raised as compared to low and marginal vitamin B12 deficiency [6].
In light of these facts, the causes for increased incidence of folate and vitamin B12 deficiency need to be elucidated. As pointed out earlier, over the last few years, vitamin B12 has taken over as a more common micronutrient deficiency as compared to folate however, the prophylaxis programs often ignore the need for vitamin B12 supplementation.
The aim of present study was to evaluate the varied clinico-haematological presentation of patients of megaloblastic anaemia associated with deficiency of vitamin B12 and folic acid because prompt diagnosis is important as it is a completely curable condition and also to prevent the complications associated with the disease.
Materials and Methods
The present cross-sectional study was carried out over a period of 18 months from September 2014 to February 2016 in the Department of Pathology, Era’s Lucknow Medical College and hospital, Lucknow, which is a tertiary care hospital.
We studied 90 cases which were grouped as follows- Group A: no deficiency; Group B: vitamin B12 deficiency; Group C: folic acid deficiency; Group D: combined deficiency.
The inclusion criteria was haemoglobin levels of anaemic patients according to WHO criteria, MCV>95fL, peripheral blood film finding- with hypersegmented neutrophils, macrocytosis [Table/Fig-1] and cases of anaemia having vitamin B12 and/or folic acid deficiency in adults with age ranging from 20 years to 65 years. All microcytic hypochromic anaemic cases having established iron deficiency exclusively or co-existing with megaloblastic anaemia, patients on vitamin B12 and/or folic acid supplementation and children having anaemia were excluded.
Peripheral smear showing macrocytes (red arrow) hypersegmented neutrophils (black arrow) (Leishman, 40X).
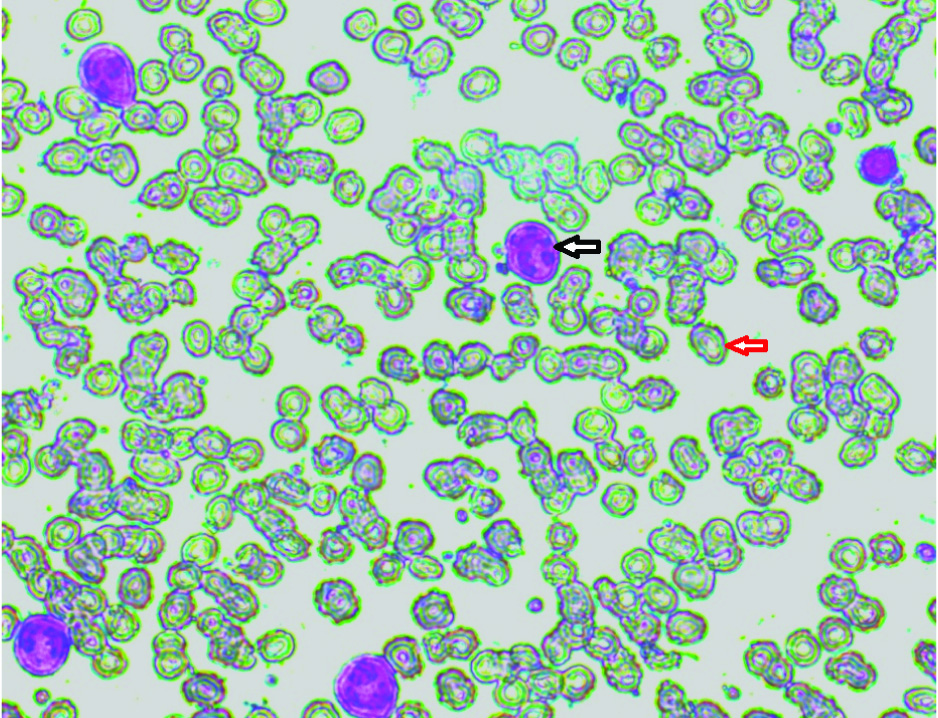
The tools of investigation were as follows: a complete blood count was obtained for all samples using Sysmex XS-800i. Smears were stained by Leishman’s stain and general blood picture of the patient was seen. Vitamin B12 Assay was done using ELISA by ACCU-BIND KIT. The test performs quantitative determination of vitamin B12 concentration in human serum by a microplate enzyme immunoassay, colorimetrically. Folic acid assessment was done using AccuDiagTM Folate ELISA Kit which employs the ELISA using sandwich complex.
Bone marrow evaluation: It was done using Salah’s needle from Posterior Superior Ileac spine using technique described by Dacie and Lewis [7].
Ethical considerations: The study was subjected to approval from Institutional Ethical Committee. Informed consent was obtained from all the participants. The participation in the study was entirely voluntary, giving the patient right to withdraw from the study whenever he/she wishes to do so.
Statistical Analysis
The data was analysed using Statistical Package for Social Sciences version 20.0. The following statistical tests were employed: mean, standard deviation, Chi-square test, Analysis of Variance (ANOVA). A value of p<0.05 was considered as significant.
Results
A total of 90 patients of macrocytosis from the Medicine outpatient and inpatient Department of Era’s Lucknow Medical College and Hospital were included in the assessment. Out of a total of 90 patients enrolled in the study, maximum (n=42; 46.7%) had vitamin B12 deficiency, followed by those having folic acid deficiency (n=19; 21.1%) and combined deficiency (n=18; 20%) respectively. A total of 11 (12.2%) did not have any deficiency.
Age of patients ranged from 20 to 65 years with mean age ranging from 34.79±12.72 years to 38.11±11.26 years. Majority of patients were males in all the groups. Statistically, there was no significant difference among groups with respect to age and gender (p>0.05) [Table/Fig-2].
Distribution of patients according to age and gender profile of patients in different groups.
| S.No | Characteristic | Group A (n=11) | Group B (n=42) | Group C (n=19) | Group D (n=18) | Statistical significance |
|---|
| 1. | Mean Age±SD (Range) in years | 37.91±14.03(20-55) | 37.48±11.83(20-64) | 34.79±12.72(20-65) | 38.11±11.26(20-60) | F=0.299; p=0.826 |
| 2. | Gender |
| Male | 8 (72.7%) | 34 (81.0%) | 13 (68.4%) | 13 (72.2%) | χ2=1.342; p=0.718 |
| Female | 3 (27.3%) | 8 (19.0%) | 6 (31.6%) | 5 (27.8%) |
In all the four groups, weakness and fatigue were most common complaints while dyspnoea and yellowish discoloration were least common. Statistically, there was no significant difference among groups with respect to complaints (p>0.05) [Table/Fig-3].
Distribution of patients in different groups for presenting complaints.
| S.No | Complaint | Group A (n=11) | Group B (n=42) | Group C (n=19) | Group D (n=18) | Statistical significance |
|---|
| No. | % | No. | % | No. | % | No. | % | χ2 | p-value |
|---|
| 1. | Weakness | 11 | 100.0 | 41 | 97.6 | 19 | 100.0 | 17 | 94.4 | 1.61 | 0.658 |
| 2. | Fatigue | 9 | 81.8 | 22 | 52.4 | 11 | 57.9 | 10 | 55.6 | 3.14 | 0.370 |
| 3. | Dyspnoea | 1 | 9.1 | 4 | 9.5 | 4 | 21.1 | 2 | 11.1 | 1.79 | 0.618 |
| 4. | Loss of appetite | 4 | 36.4 | 13 | 31.0 | 9 | 47.4 | 5 | 27.8 | 2.01 | 0.571 |
| 5. | Yellowish discoloration | 1 | 9.1 | 6 | 14.3 | 2 | 10.5 | 1 | 5.6 | 1.043 | 0.791 |
All the cases had pallor. Icterus, tingling, numbness and murmurs were relatively rare in all the groups. Hepatomegaly was seen to be affecting 15.8% to 50% patients in different groups. A statistically significant difference among groups was observed with respect to complaints like tingling and numbness [Table/Fig-4].
Distribution of patients in different groups for systemic examination findings.
| S.No | Finding | Group A (n=11) | Group B (n=42) | Group C (n=19) | Group D (n=18) | Statistical significance |
|---|
| No. | % | No. | % | No. | % | No. | % | χ2 | p-value |
|---|
| 1. | Pallor | 11 | 100 | 42 | 100 | 19 | 100 | 18 | 100 | - | - |
| 2. | Icterus | 1 | 9.1 | 6 | 14.3 | 3 | 15.8 | 1 | 5.6 | 1.238 | 0.744 |
| 3. | Hepatomegaly | 3 | 27.3 | 14 | 33.3 | 3 | 15.8 | 9 | 50.0 | 5.101 | 0.165 |
| 4. | Tingling | 0 | 0 | 17 | 40.5 | 3 | 15.8 | 7 | 38.9 | 9.414 | 0.024 |
| 5. | Numbness | 0 | 0 | 18 | 42.9 | 4 | 21.1 | 6 | 33.3 | 8.610 | 0.035 |
| 6. | Murmur | 1 | 9.1 | 7 | 16.7 | 4 | 21.1 | 0 | 0 | 4.324 | 0.229 |
Statistically, no significant difference among groups was observed with respect to any of the haematological parameters except MCV. MCV values were maximum in Group D followed by Group B, C and A respectively (p<0.001). Mean Mean Corpuscular Hemoglobin (MCH) and Mean Corpuscular Hemoglobin Concentration (MCHC) values were highest in Group D but not significant statistically. Mean neutrophil percentage were lowest in Group C but within normal range and statistically not significant. Mean lymphocyte values were higher in Groups C and D as compared to Groups A and B but difference was not significant statistically [Table/Fig-5].
Distribution of patients in different groups for haematological parameters.
| S.No | Finding | Group A (n=11) | Group B (n=42) | Group C (n=19) | Group D (n=18) | Statistical significance |
|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | F | p-value |
|---|
| 1. | Hb (gm/dL) | 10.1 | 1.6 | 9.4 | 1.7 | 9.6 | 1.8 | 9.7 | 1.7 | 0.477 | 0.699 |
| 2. | RBC | 3.2 | 0.6 | 2.76 | 0.75 | 3.04 | 0.84 | 2.75 | 0.64 | 0.903 | 0.443 |
| 3. | Hct (%) | 31.4 | 6.1 | 31.3 | 8.0 | 30.2 | 6.9 | 30.8 | 6.3 | 0.115 | 0.951 |
| 4. | MCV (fL) | 101.2 | 0.6 | 105.9 | 3.6 | 103.0 | 2.8 | 109.4 | 4.8 | 16.444 | <0.001 |
| 5. | MCH (pg) | 32.8 | 1.1 | 34.4 | 2.1 | 34.4 | 3.8 | 35.1 | 1.9 | 1.956 | 0.127 |
| 6. | MCHC (gm/dL) | 33.0 | 1.3 | 33.8 | 1.8 | 33.2 | 1.5 | 34.0 | 1.8 | 1.304 | 0.279 |
| 7. | Platelet count (103/μL) | 203.1 | 54.1 | 144.0 | 79.3 | 192.8 | 118.9 | 162.2 | 91.9 | 2.058 | 0.112 |
| 8. | TLC (103/μL) | 7.1 | 2.1 | 5.7 | 2.5 | 6.2 | 2.5 | 6.6 | 2.6 | 1.226 | 0.305 |
| 9. | DLC (%) |
| N | 74.6 | 11.2 | 74.3 | 8.3 | 70.6 | 8.5 | 71.3 | 6.2 | 1.261 | 0.293 |
| L | 18.9 | 9.4 | 20.4 | 7.5 | 23.9 | 7.0 | 23.1 | 6.0 | 1.747 | 0.163 |
| E | 4.0 | 2.2 | 3.5 | 2.1 | 3.2 | 1.6 | 3.4 | 1.3 | 0.474 | 0.701 |
| M | 1.5 | 1.0 | 1.4 | 1.2 | 1.7 | 1.1 | 1.8 | 1.1 | 0.495 | 0.686 |
N=Neutrophil, L=Lymphocyte, E=Eosinophil, M=Monocyte.
Mean vitamin B12 levels were significantly higher in Group A and Group C as compared to Group B and Group D (p<0.001). Mean folic acid levels were significantly higher in Group A and Group B as compared to that in Group C and Group D (p<0.001) [Table/Fig-6].
Comparison of patients in different groups for vitamin B12 and folic acid levels.
| S.No | Finding | Group A (n=11) | Group B (n=42) | Grop C (n=19) | Group D (n=18) | Statistical significance |
|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | F | p-value |
|---|
| 1. | Vitamin B12 (pg/mL) | 499.5 | 116.4 | 179.4 | 80.9 | 432.5 | 134.1 | 172.1 | 78.6 | 55.04 | <0.001 |
| 2. | Folic acid (ng/mL) | 5.6 | 1.3 | 6.0 | 1.4 | 3.0 | 1.4 | 2.9 | 1.4 | 35.61 | <0.001 |
All the 14 cases for which bone marrow examination [Table/Fig-7] was carried out had erythroid hyperplasia and megaloblastic reaction. Dyserythropoiesis was seen in five cases of Group B and two cases of Group D while giant metamyelocytes were seen in only six cases of Group B, two cases of Group C and four cases of Group D [Table/Fig-8].
Bone marrow smears showing megaloblasts (black arrow) and dyserythropoiesis (red arrow) (Lieshman, 40X).
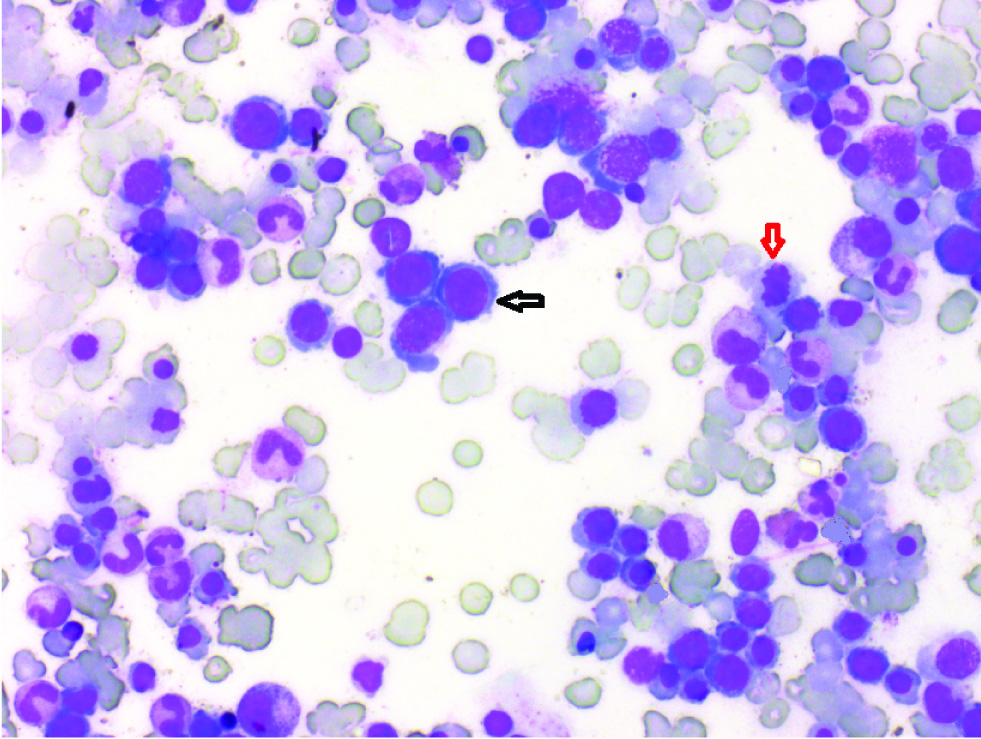
Distribution of patients in different groups on the basis of bone marrow examination (n=14).
| S.No | Conditions | Group A (n=0) | Group B (n=8) | Group C (n=2) | Group D (n=4) |
|---|
| 1. | Erythroid hyperplasia | 0 | 8 | 2 | 4 |
| 2. | Megaloblastic Reaction | 0 | 8 | 2 | 4 |
| 3. | Dyserythropoiesis | 0 | 5 | 0 | 2 |
| 4. | Giant metamyelocytes | 0 | 6 | 2 | 4 |
Discussion
Megaloblastic anaemia are characterised by the presence of megaloblasts which have a large size and specific alteration in appearance of nuclear chromatin. There is retarded DNA synthesis and RNA synthesis remains unimpaired. The most common causes of megaloblastic anaemia are vitamin B12 and folic acid deficiencies.
The profile of megaloblastic anaemia in different studies carried out in adults, show a considerable variation. Study by Calvo Romero JM et al., among elderly patients in Spain reported vitamin B12 deficiency in 80% of patients, folate deficiency in 14% patients and combined folate and vitamin B12 deficiency in 3% patients [8]. Abdulmanea AA et al., in a study of 161 patients from Saudi Arabia, reported prevalence of vitamin B12 deficiency in only 2.38% patients [9]. In contrast to this out of a total of 90 patients enrolled in the present study, maximum (n=42; 46.7%) had vitamin B12 deficiency, followed by those having folic acid deficiency (n=19; 21.1%) and combined deficiency (n=18; 20%) respectively. A total of 11 (12.2%) did not have any deficiency. In present study, weakness and fatigue were the most common presenting complaints seen in 97.6% and 57.9% patients respectively. Statistically, there was no significant difference among different diagnostic groups on this part.
With respect to different presenting signs and systemic examination findings, pallor was found to be a universal finding in all the cases. Pallor often is a generalised finding in all types of anaemia. Khanduri U and Sharma A and; Mahajan SK and Aundhakar SC reported it to be present in 85% of their cases of megaloblastic anaemia [10,11]. Unnikrishnan V et al., and Siddiqui B et al., in their study, similar to our study also found pallor to be present in 100% of their patients [12,13]. In the present study, hepatomegaly, numbness and tingling were observed in 32.2%, 31.1% and 30% patients respectively. However, among this triad, only tingling and numbness showed a significant difference among different groups. Both these findings were more common in vitamin B12 and combined deficiency as compared to folic acid deficiency. Incidentally, all the cases in non-deficiency group did not show signs of numbness and tingling.
However, Khanduri U and Sharma A, reported of presence of numbness in only 1 out of 23 cases of megaloblastic anaemia solely attributable to vitamin B12 or vitamin B12 and folate deficiency [10]. In another study, Mahajan SK and Aundhakar SC, reported presence of tingling and numbness in nearly 11% patients in a series of 100 patients in which 25% had vitamin B12 or combined vitamin B12 and folate deficiency [11].
Numbness and tingling have been reported to be the characteristic findings for identification of vitamin B12 deficiency in some case reports [14,15], however, its usefulness in ascertaining vitamin B12 as the cause of megaloblastic anaemia is not reported in any study.
On evaluating the haematological profile of patients, statistically no significant difference among different diagnostic groups was observed with respect to any of the haematological parameters except MCV values. In present study, the MCV values in vitamin B12 and combined deficiency groups were significantly higher as compared to those in no deficiency and folic acid deficiency groups. Contrary to findings of present study, Toprak B et al., reported that for low and marginal folic acid deficiency, the MCV values have been found to be relatively more raised as compared to low and marginal vitamin B12 deficiency [6]. As such none of the studies so far have shown a discriminating role of MCV values among different diagnostic subtypes of megaloblastic anaemia.
As such the degree of macrocytosis was similar in different diagnostic subtypes of megaloblastic anaemia. With respect to lymphocyte count, the present study showed an incremental trend within the no deficiency to deficiency groups. A previous study has shown an association of vitamin B12 levels with lymphocytosis among vegetarians; however, whether these findings are applicable in context with megaloblastic anaemia remains to be explored [4]. In the present study, we found mean count to be maximum among those with combined or folic acid deficiency followed by those having vitamin B12 deficiency and minimum amongst non deficiency anaemia cases and as such could not find any substantial trend as suggested in the cited study.
Hypersegmented neutrophils showed a discriminatory effect with maximum presence in combined deficiency (50%) followed by vitamin B12 deficiency (26.2%), folic acid deficiency (10.5%) and no deficiency (0%). Hypersegmented neutrophils in peripheral blood are the characteristic findings of megaloblastic anaemia [18]. Siddiqui B et al., and Nizamani GS et al., also reported presence of hypersegmented neutrophils in megaloblatic anaemia cases but did not report any association with type of deficiency [13,19]. Thompson WG et al., found a significant association between vitamin B12 deficiency and hypersegmentation and found hypersegmentation to be a sensitive indicator of vitamin B12 deficiency [20]. However, in the present study, instead of being more sensitive towards vitamin B12 deficiency, hypersegmentation showed to be more specific with a 100% specificity for non deficiency megaloblastic anaemia.
In this study, in 14 cases, bone marrow examination could be performed and confirmed megaloblastic anaemia and did not show any malignancy.
Megaloblastic anaemia can present with varied clinical manifestations. Suspicion of megaloblastic anaemia should be kept in mind by the clinicians if the signs and symptoms corresponding to megaloblastic anaemia are observed, as discussed. Prompt diagnosis is important as this anaemia can be completely cured. Follow up and diet counselling should be done properly. As such the disease can be completely cured, diagnosis and treatment should not be delayed so that further the complications can be prevented. For better management, complete clinico-haematological profile of the patient is a must so that the accurate cause can be established and complications can be prevented.
Limitation
Since our sample size was small we could not find conclusive evidence of certain haematological parameters on the basis of which vitamin B12 deficiency could be differentiated from folic acid deficiency. Bone marrow biopsy could not be done in all cases. Thus, a comprehensive approach has to be adapted.
Conclusion
On the basis of above findings, it could be concluded that there is a significant difference in the clinical presentation of patients of megaloblastic anaemia with vitamin B12 and folic acid deficiency. However, not much significant difference has been noticed in the haematological parameters of the two aetiologies except that erythropenia and macrocytosis are more marked in vitamin B12 deficient megaloblastic anaemia patients. Thus, clinical and haematological profile both should be thoroughly assessed to differentiate between vitamin B12 and folic acid deficiency.
N=Neutrophil, L=Lymphocyte, E=Eosinophil, M=Monocyte.