Dapsone, an antimicrobial and anti-inflammatory drug, has wide applications. Dapsone Hypersensitivity Syndrome (DHS) is an Adverse Drug Reaction (ADR) ranging from mild cutaneous manifestations to severe life-threatening complications. A classic triad consists of fever, skin eruption and multi-organ involvement. If left untreated, it can be fatal. Organs involved must be identified and other possible causes need to be ruled out. Along with a brief review of DHS, this report emphasizes the need for awareness about ADRs among healthcare professionals as well as patients.
We report a case of 30-year-old woman with lepromatous leprosy, started on the World Health Organisation (WHO) Multi-Drug Therapy (MDT) regimen, who presented with fever, malaise, diffuse rash with itching and epigastric discomfort that began on the 21st day of treatment. She had icterus, cervical lymphadenopathy, anaemia and deranged liver function test. Dapsone was withdrawn and intravenous dexamethasone and cefotaxime were given along with oral (antihistamines, vitamin supplements) and topical drugs. She was discharged after one week. With continuation of oral treatment and regular follow up, her recovery was complete after four weeks of treatment. As she reported early and received prompt treatment, her recovery was early with lesser morbidity.
Case Report
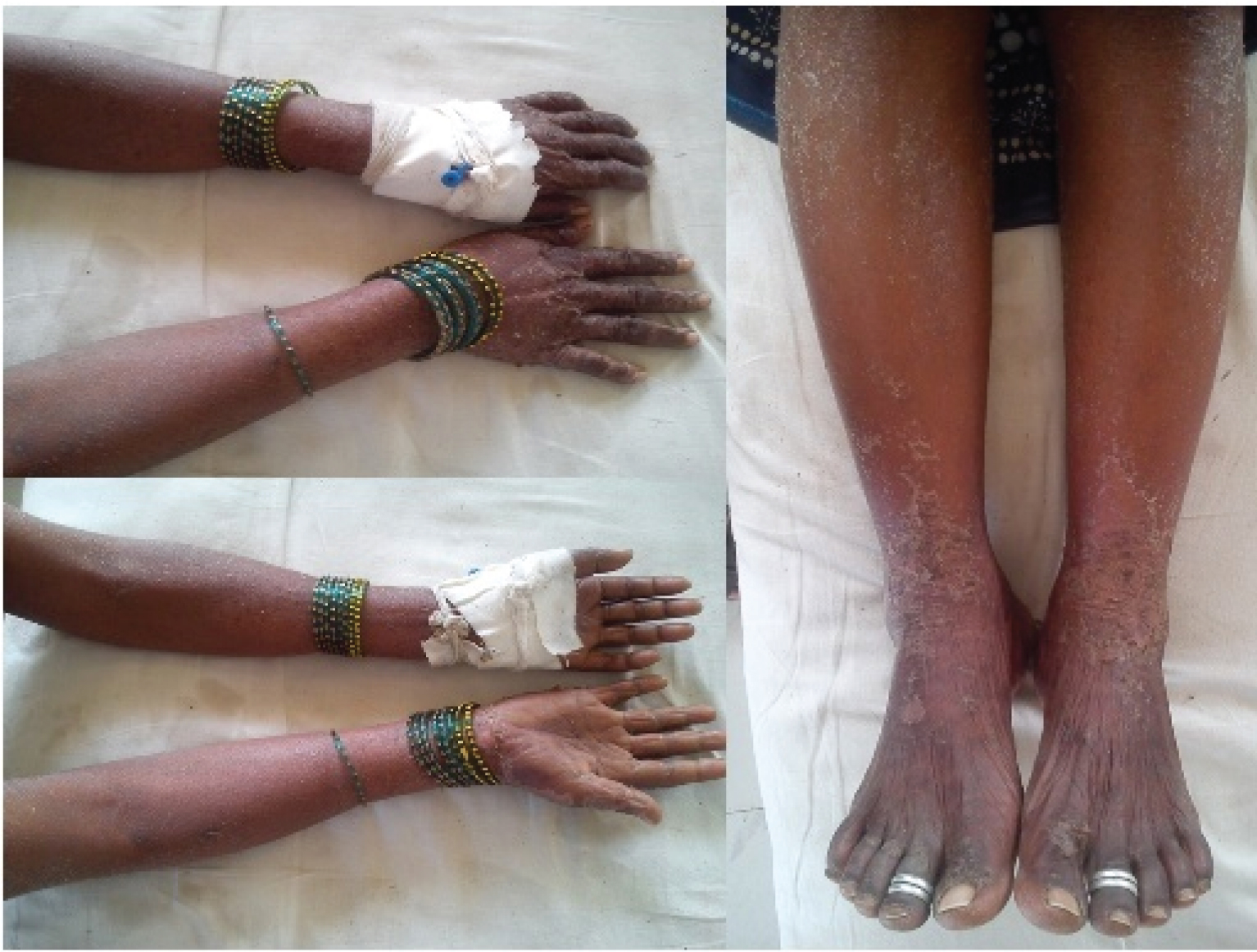
A 30-year-old female, a housewife, presented to the dermatology outpatient department, with rash and itching all over body, mild fever and malaise for two days. Twenty one days prior, she had been diagnosed with lepromatous leprosy at a Primary Health Centre and was started on World Health Organization – Multidrug Therapy (WHO-MDT) regimen for multibacillary leprosy (Rifampicin 600 mg, clofazimine 300 mg, dapsone 100 mg on day 1 and clofazimine 50 mg and dapsone 100 mg daily for 27 days: such 4 weeks given for 12 months). On enquiry, she gave history of epigastric discomfort. There was no history suggestive of nerve tenderness or aggravation of previous lesions or nodules or arthralgia. She had undergone tubal ligation three years ago. General examination revealed mild fever, pallor, icterus, and tender cervical lymph nodes. She had generalized maculopapular erythematous rash with scales [Table/Fig-1]. Eyes and mucous membranes were not involved. On per abdominal examination, she had epigastric tenderness following which she was hospitalized. The laboratory findings (complete blood counts, blood indices and liver function tests) have been summarised in [Table/Fig-2]. Peripheral smear showed hypochromic microcytic erythrocytes without any evidence of haemolysis. Liver Function Tests (LFTs) were deranged. Kidney function and serum electrolytes were within normal limits. Chest radiography and ultra-sonography of abdomen and pelvis were unremarkable.
Laboratory findings as on day 1 and day 6 of inpatient care.
| Parameter | Day 1 | Day 6 |
|---|
| Haemoglobin | 9.3 gm%, | 10.8 gm% |
| Total leucocyte count | 14,600/ mm3(neutrophil 75%, lymphocyte 18%,monocyte 5%,eosinophil 2%) | 12,400/ mm3(neutrophil 68%,lymphocyte 26%,monocyte 4%,eosinophil 2%) |
| Mean corpuscular volume | 78 fL | 83.5 fL |
| Mean corpuscular haemoglobin | 26 pg | 26.9 pg |
| Mean corpuscular haemoglobin concentration | 31.60 gm/dL | 32.22 gm/dL |
| Platelet count | 2,59,000/mm3 | 2,77,000/ mm3 |
| Erythrocyte count | 3.55 x 106/mm3 | 3.94 x 106/mm3 |
| Total bilirubin | 3.70 mg/dL | 1.1 mg/dL |
| Direct bilirubin | 1.90 mg/dL | 0.6 mg/dL |
| Indirect bilirubin | 1.80 mg/dL | 0.5 mg/dL |
| Aspartate transaminase | 306 U/L | 86 U/L |
| Alanine transaminase | 282 U/L | 80 U/L |
| Serum albumin | 2.9 gm/dL | 3.4 gm/dL |
| HBsAg | Non-reactive | -- |
| HIV | Non-reactive | -- |
The following criteria given by Richardus JH and Smith TC [1] to diagnose Dapsone Hypersensitivity Syndrome were used. These include:
The symptoms appear within eight weeks after commencement of dapsone and disappear after the discontinuation of the drug;
The symptoms cannot be ascribed to any other drug given simultaneously with dapsone;
The symptoms are not attributable to lepra reaction;
No other disease liable to cause similar symptoms is diagnosed;
Two of the following signs, symptoms should be present-fever, skin eruption, lymphadenopathy, liver pathology (hepatomegaly, jaundice and/or abnormal LFTs).
These were fulfilled in this case.
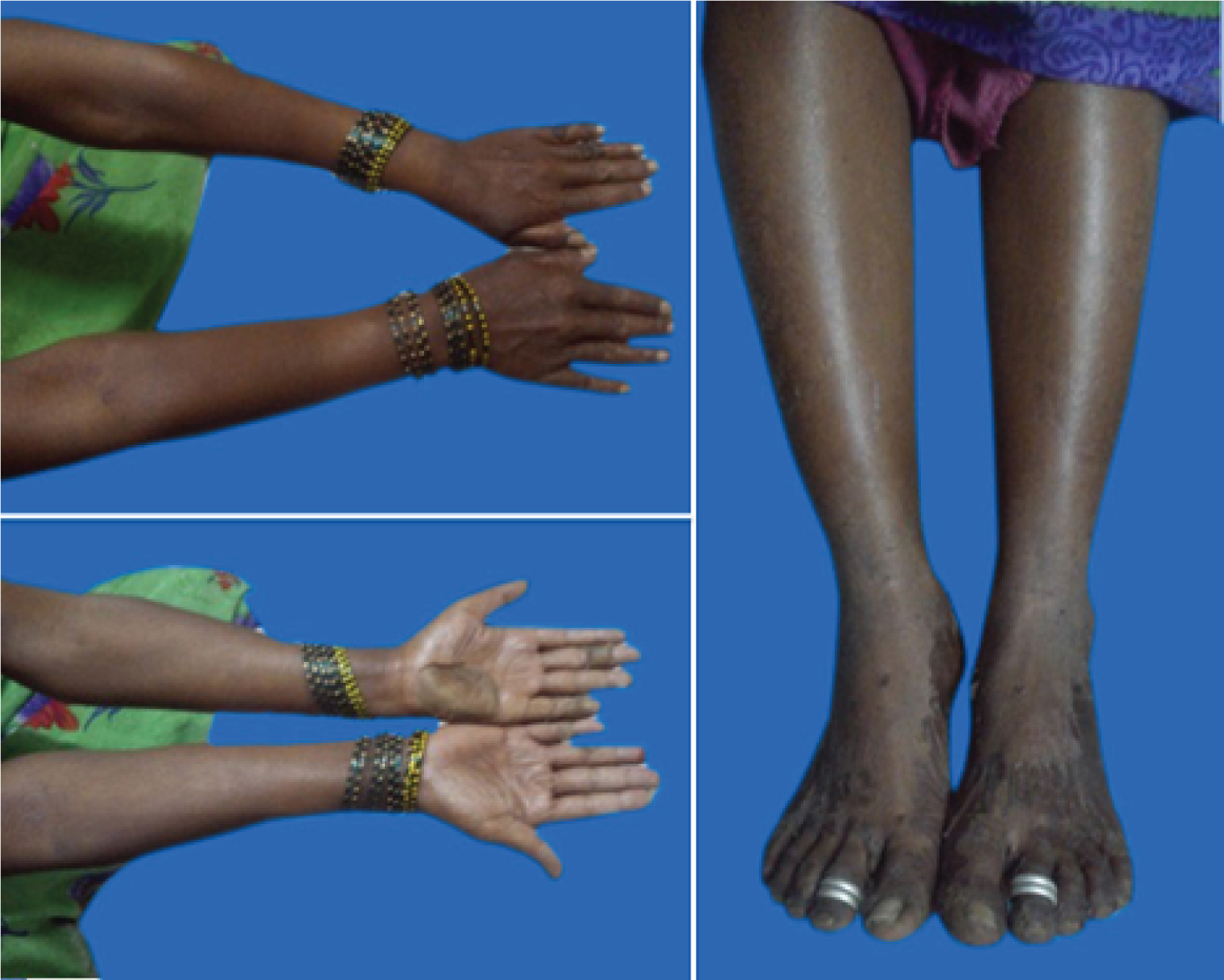
The severity of the ADR was assessed as moderate (Level 4b) according to the modified Hartwig SC and Siegel J Scale [2]. Dapsone was immediately stopped and she was given i.v. (intravenous) dexamethasone and i.v. cefotaxime (for one week), i.v. fluids, oral antihistamines, oral vitamin and mineral (calcium and iron) supplements, petroleum jelly for topical application. There was significant relief on withdrawal of dapsone. The laboratory findings of the sixth day are as shown in [Table/Fig-2]. She was discharged on seventh day of inpatient care [Table/Fig-3]. She was switched to oral steroids (dexamethasone, gradually tapered off over six weeks) and with regular follow up, recovery was complete at four weeks. Rifampicin and Clofazimine were continued. This case of ADR was reported during the pharmacovigilance activity under the Pharmacovigilance Programme of India (PvPI).
Discussion
Dapsone (4,4’- diaminodiphenylsulfone) has been in clinical use for more than 60 years. Dapsone is useful in a wide set of dermatological diseases and also in infectious diseases (namely Pneumocystis carinii pneumonia and malaria) [3]. Dapsone can cause gastric intolerance, headache, insomnia, blurred vision, paresthesias, drug fever, haematuria, pruritus, methemoglobinemia, haemolysis, and hepatitis and DHS. Toxicities include agranulocytosis, reversible peripheral neuropathy and psychosis [4].
Dapsone Hypersensitivity Syndrome (DHS)
It was first noted by Lowe in 1949 in Nigerian leprosy patient [5] and Allday and Barnes coined the term ‘Dapsone Hypersensitivity Syndrome’ in 1951 [6]. Other names include ‘dapsone syndrome’ or ‘sulfone syndrome’. It usually develops within 2–8 weeks after taking dapsone [1,7]; hence called ‘fifth-week dapsone dermatitis’. The classic triad consists of fever, rash, and internal organ involvement (most commonly liver). Hepatitis, exfoliative dermatitis, lymphadenopathy and haemolytic anaemia might be seen in varying combinations and sequences [7].
The incidence of DHS ranges from 0.5%–3.6% and has increased in the past three decades worldwide [1]. The possible contributing reasons could be combination of dapsone with other anti-leprosy drugs (particularly rifampicin), changes in its manufacturing, and increasing use in chemoprophylaxis of Pneumocystis carinii infection [1,7]. The overall case fatality is reported to be 9.9% [8].
The exact pathophysiological mechanism underlying DHS is unclear. Three hypotheses have been suggested: an immune humoral response, a delayed hypersensitivity reaction and altered hepatic metabolism including acetylation and hydroxylation with secondary toxic metabolite production [9,10]. The formation of toxic intermediate metabolites, through N-hydroxylation pathway, is thought to be responsible for the haemolytic anaemia, methemoglobinemia and also dapsone syndrome [11].
Cutaneous manifestations may occur in the form of erythroderma, maculopapular eruption, erythema multiforme, Toxic Epidermal Necrolysis (TEN) or Stevens-Johnson Syndrome (SJS) [1]. Severity of skin symptoms and severity of internal organ involvement may not correlate. Besides liver, there are reports of other internal organ involvement, such as kidneys, heart, lungs or pancreas, which may be present as additional complications [8]. Leta GC et al., classified DHS into complete and incomplete [11]. The complete form is characterized by the presence of rash, fever, lymphadenopathy, hepatomegaly and clinical or laboratory evidence of hepatic dysfunction. When one of these findings is absent, it is classified as incomplete. Most patients have a complete DHS and are often paucibacillary.
Risk Factors
Patients with viral hepatitis (HBsAg) are at increased risk for the development of DHS [12]. HLA-B*13:01 is a risk factor of DHS. Among the nations having high incidence of leprosy, India has the highest recorded prevalence of the HLA-B*13:01 allele [13]. Mucosal involvement, hepatitis, immunodeficiency diseases, delayed cessation of drug/delayed presentation, acute clinical course, leprosy as indication for dapsone use and disease occurrence in non-affluent countries are all associated with higher risk of fatal outcome. It is speculated that ageing and pre-existing liver disease may offer protection against adverse effects because of decreased enzyme activity and, therefore, decreased production of toxic metabolites [7,8,11].
Differential Diagnosis
Conditions having features common with DHS need to be distinguished for appropriate management. A few distinguishing and predominant clinical features of each are discussed in [14-16] [Table/Fig-4].
| Differential Diagnosis | Distinguishing Features/Significance |
|---|
| DRESS* (DIHS†) | DHS‡ has been considered as a variant of DRESS. Drugs incriminated include allopurinol, sulfonamides, anticonvulsants (barbiturates, phenytoin, carbamazepine, lamotrigine), and dapsone. Although their proposed aetiopathogenesis is not the same, the management is similar [7,9,14]. Leukocyte abnormalities’ (eosinophilia, presence of atypical lymphocytes, leukocytosis) forms an important diagnostic criteria for DRESS [14]. |
| Lepra Reaction | Aggravation of existing lesions, ulceration, and nerve involvement are typical findings. |
| SJS§/TEN|| | It may overlap with DHS. It is important to determine its cause and know whether it is a part of DHS. Peeling of skin (Nikolsky’s sign) with epidermal necrosis are characteristic features. |
| Infectious-mononucleosis | It is primarily a disease of young adults and predominantly presents with prolonged fever, malaise, fatigue, arthralgias or myalgias, lymphadenopathy which contrasts the characteristic cutaneous involvement in DHS. DHS has been called infectious-mononucleosis like syndrome. |
| Hypersensitivity to other concomitant drugs (particularly in leprosy) | Clofazimine is known to cause icthyosis and skin rash. Exfoliative dermatitis has also been reported [15]. Reported cases of hypersensitivity to rifampicin include flu-like syndrome, thrombocytopenia, haemolytic anaemia, renal failure and anaphylaxis [16]. |
DRESS* - Drug Reaction With Eosinophilia and Systemic Symptoms, DIHS† - Drug induced Hypersensitivity Syndrome, DHS‡ - Dapsone Hypersensitivity Syndrome, SJS§ - Stevens-Johnson syndrome, TEN|| - Toxic Epidermal Necrolysis
Management of DHS
Management constitutes immediate cessation of the offending drug, early systemic corticosteroid therapy, identification of organs involved, skin care and supportive management. The patient needs to be followed up for risk of relapse (as drug tends to be retained in skin, muscles, liver and kidney for upto three weeks [3]) after discontinuation of steroids and also for alternative treatment of the primary indication for use of dapsone. Hypothyroidism occurring after three months of dapsone therapy is well-known and is important to be ruled out [17].
Screening for HbsAg, HIV and HLA-B*13:01 before commencement of therapy, wherever possible, can be helpful in determining individuals at risk. The potential for cross-reactivity between dapsone and sulfonamides, given the sulfa moiety in dapsone, should be borne in mind and these drugs should be avoided in such patients. Genetic factors have been implicated in the aetiopathogenesis of DHS and the relatives of the patient need to be cautioned regarding the risks of using dapsone. Such counseling was done in our case.
Conclusion
Dapsone finds use in many immunological, inflammatory, dermatological disorders and insect bites and its uses are predicted to increase. The incidence of DHS is reportedly rising. It is therefore prudent to be aware of this life-threatening condition and distinguish it from other disorders with similar features. For early diagnosis and treatment of ADRs, awareness among healthcare professionals as well as patients is important. Early reporting leads to prompt intervention resulting in early recovery, thus reducing preventable drug-induced morbidity.
[1]. Richardus JH, Smith TC, Increased incidence in leprosy of hypersensitivity reactions to dapsone after introduction of multidrug therapyLepr Rev 1989 60(4):267-73. [Google Scholar]
[2]. Hartwig SC, Siegel J, Schneider PJ, Preventability and severity assessment in reporting adverse drug reactionsAmerican Journal of Health-System Pharmacy 1992 49(9):2229-32. [Google Scholar]
[3]. Gumbo T, Chemotherapy of Tuberculosis, Mycobacterium Avium Complex Disease, and Leprosy. In: Brunton LL, Chabner BA, Knollman BCGoodman and Gilman’s The Pharmacological Basis of Therapeutics 2011 12th edNew YorkMc-Graw Hill:1563-64. [Google Scholar]
[4]. Burkhart C, Morrell D, Goldsmith L, Dermatological Pharmacology. In: Brunton LL, Chabner BA, Knollman BCGoodman and Gilman’s The Pharmacological Basis of Therapeutics 2011 12th edNew YorkMc-Graw Hill:1823-24. [Google Scholar]
[5]. Lowe J, Smith M, The chemotherapy of leprosy in Nigeria with an appendix on glandular fever and exfoliative dermatitis precipitated by sulfonesInt J Lepr 1949 17(3):181-95. [Google Scholar]
[6]. Allday EJ, Barnes J, Toxic effects of diaminodiphenyl sulphone in leprosyLancet 1951 2:205-06. [Google Scholar]
[7]. Kosseifi SG, Guha B, Nassour DN, Chi DS, Krishnaswamy G, The Dapsone Hypersensitivity Syndrome revisited: A potentially fatal multisystem disorder with prominent hepatopulmonary manifestationsJ Occup Med Toxicol 2006 1:9 [Google Scholar]
[8]. Lorenz M, Wozel G, Schmitt J, Hypersensitivity reactions to dapsone: a systematic reviewActa Derm Venereol 2012 92(2):194-99. [Google Scholar]
[9]. Gavilanes MC, Palacio AL, Chellini PR, da Costa Nery JA, Rego JG, Dapsone hypersensitivity syndrome in a lepromatous leprosy patient – A Case ReportLepr Rev 2015 86(2):186-90. [Google Scholar]
[10]. Prussick R, Shear NH, Dapsone hypersensitivity syndromeJ Am Acad Dermatol 1996 35(2 Pt 2):346-49. [Google Scholar]
[11]. Leta GC, Simas MEP, Oliveira MLW, Gomes MK, Dapsone hypersensitivity syndrome: a systematic review of diagnostic criteriaHansenol Int 2003 28(1):79-84. [Google Scholar]
[12]. Pavithran K, Bindu V, Dapsone syndrome: hepatitis-B infection a risk factor for its development?Int J Lepr Other Mycobact Dis 1999 67(2):171-72. [Google Scholar]
[13]. Zhang FR, Liu H, Irwanto A, Fu XA, Li Y, Yu GQ, HLA-B*13:01 and the dapsone hypersensitivity syndromeN Engl J Med 2013 369(17):1620-28. [Google Scholar]
[14]. Criado PR, Criado RFJ, Avancini JM, Santi CG, Drug reaction with Eosinophilia and Systemic Symptoms (DRESS)/Drug-Induced Hypersensitivity Syndrome (DIHS): a review of current conceptsAn Bras Dermatol 2012 87(3):435-49. [Google Scholar]
[15]. Pavithran K, Exfoliative dermatitis after clofazimineInt J Lepr Other Mycobact Dis 1985 53(4):645-46. [Google Scholar]
[16]. Martinez E, Collazos J, Mayo J, Hypersensitivity Reactions to Rifampin. Pathogenetic mechanisms, Clinical Manifestations, Management Strategies, and Review of the Anaphylactic-like ReactionsMedicine 1999 78(6):361-9. [Google Scholar]
[17]. Gupta A, Eggo MC, Uetrecht JP, Cribb AE, Daneman D, Rieder MJ, Drug-induced hypothyroidism: The thyroid as a target organ in hypersensitivity reactions to anticonvulsants and sulfonamidesClin Pharmacol Ther 1992 51(1):56-67. [Google Scholar]