Budesonide Nasal Douching: An Effective Method in Postoperative AFRS Management
Neena Chaudhary1, Shashank Gupta2, Rajeev Kumar Verma3, Santosha Ram Choudhary4
1 Professor, Department of Otorhinolaryngology, VMMC and Safdarjung Hospital, Delhi, India.
2 Junior Resident, Department of Otorhinolaryngology, VMMC and Safdarjung Hospital, Delhi, India.
3 Senior Resident, Department of Otorhinolaryngology, VMMC and Safdarjung Hospital, Delhi, India.
4 Senior Resident, Department of Otorhinolaryngology, VMMC and Safdarjung Hospital, Delhi, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Rajeev Kumar Verma, Krashi Upaz Mandi Road, Ward No 1, Shrimadhopur, Siker-332715, Rajasthan, India.
E-mail: drrajeevkumar85@gmail.com
Introduction
The aim of management of Allergic Fungal Rhinosinusitis (AFRS) is to control the disease process and the local immune response of the nasal mucosa. This is achieved by a two pronged approach – surgical clearance of all sinuses by Functional Endoscopic Sinus Surgery (FESS) followed by suppression of local immune response using steroids.
Recently delivery of topical steroid by way of douching has been introduced. The effect of budesonide in management of postoperative AFRS patients is yet to be studied.
Aim
To evaluate the role of budesonide nasal douching in postoperative management of AFRS.
Materials and Methods
A total of 60 postoperative AFRS patients were randomly divided into two groups. Both groups received routine post Functional Endoscopic Sinus Surgery (FESS) medication as per the institute protocol. One group of patients received budesonide douching in addition to the routine care. Both groups were evaluated endoscopically at one, two and six weeks post op. Pre and postoperative quality of life change, patient complains, need for revision surgery and endoscopic Kupferberg scoring were used to compare the two postoperative groups.
Results
The average preoperative Sino-Nasal Outcome Test 22 (SNOT-22) score was 50.2. It was reduced to an average of 29.6 in patients who used the standard postoperative regimen and to 19.8 postoperatively in patients who had budesonide added to their douching solutions.
The average Kupferberg score was 1.2 for patients who did receive budesonide as compared to 1.9 for patients who did not receive budesonide douching.
Conclusion
Budesonide douching can offer a safe and effective tool in managing local inflammatory response in AFRS. It leads to a significantly better quality of life and has an effective response on nasal mucosa – leading to lesser mucosal oedema and lesser incidence of polypoidal changes postoperatively.
Allergic fungal rhinosinusitis, Douching, Functional endoscopic sinus surgery
Introduction
The discovery of AFRS can be traced back to 1971 when McCarthy and Pepys observed that 10% of patients with Allergic Broncho-Pulmonary Aspergillosis (ABPA) also had symptoms of nasal obstruction due to plugs that were similar to the mucous plugs in their respiratory tract [1]. Culture of these plugs and samples of antral lavage grew Aspergillus fumigatus. Katzenstein et al., described the presence of “allergic mucin” in patients with Asthma, Nasal polyposis and Sinusitis [2]. They called the condition “allergic aspergillus sinusitis” and found that the allergic mucin contained laminated mucin, eosinophils, eosinophil Charcot-Leyden crystals, and fungal hyphae.
Since then a lot of research has been carried out in what is now called AFRS. Various diagnostic criteria have also been laid down for it. The most accepted criteria has been given by Bent III JP and Kuhn FA [Table/Fig-1] [3] and has been used in this study.
Bent and Kuhn Criteria for diagnosis of AFRS [3].
| Major Criteria | Minor Criteria |
|---|
| Type 1 Hypersensitivity | Asthma |
| Nasal polyposis | Unilateral disease |
| Characteristic CT findings | Bone erosion |
| Eosinophilic mucin without invasion | Charcot-Leyden crystals |
| Positive Fungal Stain | Serum Eosinophilia |
| CT, Computed Tomography | Fungal culture positive |
Effective control of the disease process relieves symptoms, improves quality of life and reduces recurrence of disease. Management includes a regimen of oral corticosteroids, topical steroid, nasal spray and hypertonic saline nasal douching.
Lately, there is increasing evidence that sprays and aerosols are not able to effectively reach the paranasal sinuses, and that, in most cases, these products do not even reach the middle meatus area.
In such a scenario, these low volume high pressure methods should be disregarded in favour of high-volume methods [4,5], with a daily irrigation of atleast 200 mL [6]. In view of evidence of better efficacy of high volume/low pressure solutions with respect to sinus penetration, nasal irrigation with corticosteroids have been used with encouraging results [7]. In this line, subgroups traditionally more difficult to control (e.g. Chronic Rhinosinusitis with polyps and increased tissue eosinophils) have better therapeutic response than the other subgroups [8].
Recently, delivery of topical steroid by way of douching has been introduced. One of the drugs used for instillation in topical forms is budesonide. Budesonide, at daily doses ranging from 250μg to 1 mg has been used [8]. Budesonide is a potent topical corticosteroid with an approximately 1,000-fold higher topical anti-inflammatory potency than cortisol. Budesonide binds the glucocorticoid receptor and exerts an anti- inflammatory effect through several mechanisms including altering the release of arachidonic acid metabolites, inhibiting the accumulation of leukocytes in a resected tissue, decreasing vascular permeability, inhibiting neuropeptide mediated responses, and altering the secretion of glycoproteins from sub-mucosal glands.
This study aims at evaluating the role of budesonide nasal douching as a tool for controlling local inflammation in post-FESS patients of AFRS.
Materials and Methods
This retrospective study was carried out at Department of ENT, VMMC & Safdarjung Hospital, New Delhi, India – a tertiary care hospital in north India between July 2014-December 2016. Ethical committee approval and fully informed patient consent were obtained.
A 60 post-FESS patients of AFRS [Table/Fig-2,3] who were operated for nasal polyposis were included in this study and were divided into cases and controls. They were included irrespective of their age and sex. Patients with comorbidities like diabetes mellitus, hypertension, or any other condition that prevented the use of standard postoperative protocol were not included in the study.
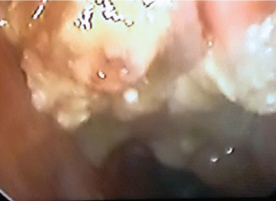
Nasal polyps with fungal debris and discharge. Patient diagnosed as suffering from AFRS. Right sided viewed with a 0-degree endoscope.
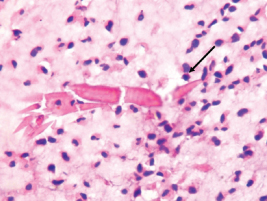
Microscopic image showing “Charcot Leyden Crystals” (Black Arrow) – a minor criteria for diagnosing AFRS. H&E staining. 100X Magnification.
The standard treatment protocol at our center is to start the patient on oral Prednisolone 1 mg/kg one week prior to surgery which is then continued till one week post-op and then gradually tapered. Patients were also started on Fluticasone nasal spray and hypertonic saline nasal douching after nasal pack removal. Oral antibiotics are continued for one week postoperatively.
In addition to this regimen, we added budesonide nasal douching to the regimen of every alternate patient (i.e., 30 patients) that was operated. About 1 mg of budesonide was mixed in 500 mL normal saline and the solution was used for douching – half in the morning and half in the evening. Budesonide douching continued for a period of six weeks postoperatively. Since, budesonide was used in 1 mg/500 mL (0.002 mg/mL) concentration, same strength solution was used irrespective of age.
Patients were assessed endoscopically at one, six and 10 weeks postoperatively. SNOT-22 [Table/Fig-4] scoring was carried out during preoperative workup and at 10 weeks postoperative for both cases and controls. SNOT-22 is a subjective scoring form filled by the patient, describing their symptoms and their severity. It is a patient centered scoring method of evaluating how the patients perceive the symptoms and any relief or exaggeration experienced by them. Endoscopic evaluation of nasal mucosa was done using the Kupferberg [9] postoperative nasal mucosa endoscopic score [Table/Fig-5,6] at 10 weeks [Table/Fig-7]. Kupferberg scoring is an objective method of evaluating the status of the nasal mucosa. It is simple, accurate and reflects the status of the AFRS disease process.
| No Problem | Very Mild Problem | Mild or slight Problem | Moderate Problem | Severe Problem | Problem as bad as it can be | 5 Most Important Items |
|---|
| 1. Need to blow nose | 0 | 1 | 2 | 3 | 4 | 5 | - |
| 2. Nasal Blockage | 0 | 1 | 2 | 3 | 4 | 5 | ○ |
| 3. Sneezing | 0 | 1 | 2 | 3 | 4 | 5 | ○ |
| 4. Runny nose | 0 | 1 | 2 | 3 | 4 | 5 | ○ |
| 5. Cough | 0 | 1 | 2 | 3 | 4 | 5 | ○ |
| 6. Post-nasal discharge | 0 | 1 | 2 | 3 | 4 | 5 | - |
| 7. Thick nasal discharge | 0 | 1 | 2 | 3 | 4 | 5 | ○ |
| 8. Ear fullness | 0 | 1 | 2 | 3 | 4 | 5 | ○ |
| 9. Dizziness | 0 | 1 | 2 | 3 | 4 | 5 | ○ |
| 10. Ear pain | 0 | 1 | 2 | 3 | 4 | 5 | ○ |
| 11. Facial pain/pressure | 0 | 1 | 2 | 3 | 4 | 5 | - |
| 12. Decreased Sense of Smell/Taste | 0 | 1 | 2 | 3 | 4 | 5 | ○ |
| 13. Difficulty falling asleep | 0 | 1 | 2 | 3 | 4 | 5 | ○ |
| 14. Wake up at night | 0 | 1 | 2 | 3 | 4 | 5 | ○ |
| 15. Lack of a good night’s sleep | 0 | 1 | 2 | 3 | 4 | 5 | ○ |
| 16. Wake up tired | 0 | 1 | 2 | 3 | 4 | 5 | - |
| 17. Fatigue | 0 | 1 | 2 | 3 | 4 | 5 | ○ |
| 18. Reduced productivity | 0 | 1 | 2 | 3 | 4 | 5 | ○ |
| 19. Reduced concentration | 0 | 1 | 2 | 3 | 4 | 5 | ○ |
| 20. Frustrated/restless/irritable | 0 | 1 | 2 | 3 | 4 | 5 | ○ |
| 21. Sad | 0 | 1 | 2 | 3 | 4 | 5 | - |
| 22. Embarrassed | 0 | 1 | 2 | 3 | 4 | 5 | ○ |
“All rights reserved. Copyright 2006. Washington University in St. Louis, Missouri.”
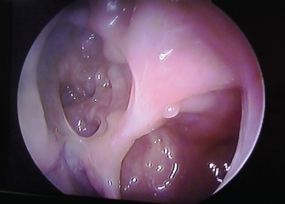
Postopertive image of clear sinuses (with budesonide douching). Left side view with) -degree endoscope showing maxillary and ethmoid sinuses.
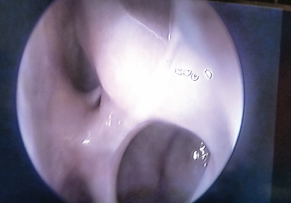
Postopertive image of clear maxillary and ethmoid sinus (without budesonide douching). Right sided view with zero degree endoscope showing ethmoid sinuses.
Kupferberg Postopertive Endoscopic Scoring [9].
| Grade | Endoscopic Findings |
|---|
| 0 | No mucosal oedema with no allergic mucin |
| 1 | Mucosal oedema with or without allergic mucin |
| 2 | Polypoidal oedema with or without allergic mucin |
| 3 | Sinus polyps with fungal debris/allergic mucin |
Therefore, both subjective and objective criteria were used to compare the effects of budesonide nasal douching. The data was compared using student’s t-test and Chi-square tests as applicable.
Results
The average age of the patients was 34.3 years with a male to female ratio of 1:1.6. The clinical features of all the patients are summarised in [Table/Fig-8].
Clinical features of all patients in the study.
| Characterisitc | Number of patients (%) |
|---|
| Nasal Polyps | 60 (100%) |
| Type 1 Hypersensitivity | 42 (70%) |
| Characteristic CT findings | 37 (61.7%) |
| Eosinophilic Mucin | 28 (46.7%) |
| Positive Fungal Stain | 11 (18.3%) |
| Asthma | 32 (53.3%) |
| Unilateral disease (AFRS) | 28 (46.7%) |
| Bone erosion present on CT scan | 8 (13.3%) |
| Fungal culture positive | 9 (15%) |
| Charcot-Leyden crystals | 16 (26.7%) |
| Serum Eosinophilia | 25 (41.7%) |
The average preoperative SNOT-22 score was 50.2. It was reduced to an average of 29.6 in patients who used the standard postoperative regimen and to 19.8 postoperatively in patients who had budesonide added to their douching solutions. This difference was found to be statistically significant (p-value-0.02) [Table/Fig-9].
| SNOT-22 Score |
|---|
| Preoperative | 50.2 |
| Postop Without budesonide | 29.6 |
| Postop With budesonide | 19.8 |
Two patients in the case group and two in the control group developed recurrence of disease and required revision surgery.
The average Kupferberg score was 1.2 for patients who received budesonide as compared to 1.9 for patients who did not receive budesonide douching. This difference was however not found to be statistically significant (p-value-0.10) [Table/Fig-10,11].
| Average Kupferberg Scores |
|---|
| Postop without budesonide | 1.9 |
| Postop with budesonide | 1.2 |
Postopertive Kupferberg scores of patients who received budesonide and those who didn’t.
| Kupferberg Scores | Post-op without Budesonide | Post-op with Budesonide |
|---|
| 0 | 2 | 8 |
| 1 | 7 | 11 |
| 2 | 13 | 8 |
| 3 | 8 | 3 |
Discussion
The safety and usefulness of this method of delivering topical steroids has been studied in CRS and been accepted by many physicians [8,11-13]. Various authors have used budesonide solutions of different strengths to good effect in managing Chronic Rhinosinusitis. Seiberling et al., used 0.25 mg budesonide per day [14], Welch et al., used 2 mg/day while Bhalla used 1 mg/day for nasal douching by mixing respules of budesonide in normal saline [10,15]. All authors have achieved varying degrees of success using topical steroid douching in controlling nasal mucosa inflammation.
In our knowledge, this is the first study aimed at evaluating the role of topical budesonide therapy in managing postoperative AFRS cases. Our study shows that budesonide douching can offer a safe and effective tool in managing local inflammatory response in AFRS. It leads to a significantly better quality of life and has an effective response on nasal mucosa – leading to lesser mucosal oedema and lesser incidence of polypoidal changes postoperatively.
Limitation
This study has certain limitations. A larger sample size is required before definitive recommendations can be made. Also, a longer follow-up period is required to completely evaluate the efficacy of budesonide douching.
Conclusion
On the basis of our findings, we recommend that budesonide nasal douching is an effective and useful adjunctive therapy in postoperative management of AFRS patients. It provides a better quality of life and is helpful in controlling the disease progression.
Future larger controlled trials are needed to improve the level of evidence for effectiveness and to assess the safety of higher doses and longer-term therapy of budesonide irrigations in patients with AFRS.
“All rights reserved. Copyright 2006. Washington University in St. Louis, Missouri.”
[1]. McCarthy DS, Pepys J, Allergic broncho-pulmonary aspergillosisClin Allergy 1971 1:261-86. [Google Scholar]
[2]. Katzenstein AL, Sale SR, Greenberger PA, Allergic Aspergillus Sinusitis A newly recognized form of sinusitisJ Allergy Clin Immunol 1983 72(1):82-93. [Google Scholar]
[3]. Bent III JP, Kuhn FA, Diagnosis of allergic fungal sinusitisOtolaryngology. Head and Neck Surgery 1994 111(5):580-88. [Google Scholar]
[4]. Wormald PJ, Cain T, Oates L, Hawke L, Wong I, A comparative study of three methods of nasal irrigationLaryngoscope 2004 114(12):2224-27. [Google Scholar]
[5]. Abadie WM, McMains KC, Weitzel EK, Irrigation penetration of nasal delivery systems: a cadaver studyInt Forum Allergy Rhinol 2011 1(1):46-49. [Google Scholar]
[6]. Rudmik L, Hoy M, Schlosser RJ, Harvey RJ, Welch KC, Lund V, Topical therapies in the management of chronic rhinosinusitis - an evidence-based review with recommendationsInt Forum Allergy Rhinol 2013 3(4):281-98. [Google Scholar]
[7]. Steinke JW, Payne SC, Tessier ME, Borish LO, Han JK, Borish LC, Pilot study of budesonide inhalant suspension irrigations for chronic eosinophilic sinusitisJ Allergy Clin Immunol 2009 124(6):1352-54.e7. [Google Scholar]
[8]. Snidvongs K, Pratt E, Chin D, Sacks R, Earls P, Harvey RJ, Corticosteroid nasal irrigations after endoscopic sinus surgery in the management of chronic rhinosinusitisInt Forum Allergy Rhinol 2012 2(5):415-21. [Google Scholar]
[9]. Kupferberg SB, Bent JP, Kuhn FA, Prognosis for allergic fungal sinusitisOtolaryngol Head Neck Surg 1997 117(1):35-41.PubMed PMID: 9230320 [Google Scholar]
[10]. Welch KC, Thaler ER, Doghramji LL, Palmer JN, Chiu AG, The effects of serum and urinary cortisol levels of topical intranasal irrigations with budesonide added to saline in patients with recurrent polyposis after endoscopic sinus surgeryAm J Rhinol Allergy 2010 24(1):26-28. [Google Scholar]
[11]. Sachanandani NS, Piccirillo JF, Kramper MA, Thawley SE, Vlahiotis A, The effect of nasally administered budesonide respules on adre- nal cortex function in patients with chronic rhinosinusitisArch Otolaryngol Head Neck Surg 2009 135(3):303-07. [Google Scholar]
[12]. Thamboo A, Manji J, Szeitz A, Santos RD, Hathorn I, Gan EC, The safety and efficacy of short-term budesonide delivered via mucosal atomization device for chronic rhinosinusitis without nasal polyposisInt Forum Allergy Rhinol 2014 4(5):397-402. [Google Scholar]
[13]. Jang DW, Lachanas VA, Segel J, Kountakis SE, Budesonide nasal irrigations in the postoperative management of chronic rhinosinusitisInt Forum Allergy Rhinol 2013 3(9):708-11. [Google Scholar]
[14]. Seiberling KA, Chang DF, Nyirady J, Park F, Church CA, Effect of intranasal budesonide irrigations on intraocular pressureInt Forum Allergy Rhinol 2013 3(9):704-07. [Google Scholar]
[15]. Bhalla RK, Payton K, Wright ED, Safety of budesonide in saline sinonasal irrigations in the management of chronic rhinosinusitis with polyposis: lack of significant adrenal suppressionJ Otolaryngol Head Neck Surg 2008 37(6):821-25. [Google Scholar]