Prosthetic Joint Infection due to Burkholderia cenocepacia: An Opportunistic Pathogen with an Expanding Spectrum of Disease
Sujeesh Sebastian1, Rajesh Malhotra2, Rojaleen Das3, Arti Kapil4, Benu Dhawan5
1 PhD Scholar, Department of Microbiology, All India Institute of Medical Sciences, Delhi, India.
2 Professor and Head, Department of Orthopaedics, All India Institute of Medical Sciences, Delhi, India.
3 Senior Resident, Department of Microbiology, All India Institute of Medical Sciences, Delhi, India.
4 Professor, Department of Microbiology, All India Institute of Medical Sciences, Delhi, India.
5 Professor, Department of Microbiology, All India Institute of Medical Sciences, Delhi, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Benu Dhawan, Room No. 2075, Department of Microbiology, All India Institute of Medical Sciences, Ansari Nagar-110029, Delhi, India.
E-mail: dhawanb@gmail.com
Burkholderia cenocepacia is an opportunistic pathogen widespread in moist environments. It has been associated with lung infections, blood, skin and genitourinary tract infections. We report here the first case of Prosthetic Joint Infection (PJI) caused by B. cenocepacia isolated from the periprosthetic tissue samples and prosthesis sonicate fluid identified by Matrix-Assisted Laser Desorption Ionization-Time-of-Flight Mass Spectrometry (MALDI-TOF MS). A good clinical outcome was obtained by two-stage exchange arthroplasty and administration of co-trimoxazole and ciprofloxacin. In addition to the expanding spectrum of this opportunistic pathogen, this case also shows the reliability of newer diagnostic tools to rapidly identify the Burkholderia Cepacia Complex (BCC) to the species level.
Burkholderia cepacia complex, Mass spectrometry, Periprosthetic tissue
Case Report
An 85-year-old male presented to the orthopaedic outpatient department with a one-year history of severe pain and discharging sinus from the left knee. He had undergone an uncomplicated bilateral Total Knee Arthroplasty (TKA) for bilateral osteoarthritis of knees eight-years back. Two-years later, he was readmitted with severe left knee pain following a trivial fall at home. Radiographs revealed periprosthetic fracture and dislocation of prosthesis. The patient underwent implant removal and left knee revision arthroplasty. The patient remained symptom free for four years. Subsequently, infection ensued and he developed a discharging sinus over the medial aspect of the left knee accompanied with severe left knee pain. The patient was immunocompetent without cystic fibrosis. He had a history of Coronary Artery Disease (CAD), diabetes mellitus and hypertension. On physical examination, the left knee had swelling and tenderness with a painful range of motion. Laboratory investigations revealed haemoglobin 67 g/dL, a Total Leukocyte Count (TLC) of 8,500/mm3, Erythrocyte Sedimentation Rate (ESR) of 43 mm/hr and C-Reactive Protein (CRP) level of 33.6 mg/L. Radiographs of the left hip revealed loosening of the prosthesis.
The patient underwent arthocentesis of the left knee joint and the aspirate was sent for microbiological investigations. Gram stain showed numerous polymorphonuclear leucocytes but no organisms. A 10% Potassium Hydroxide (KOH) mount was negative for fungal elements. Both bacterial and fungal cultures were sterile. The patient was subsequently posted for a two stage revision knee replacement. The first stage involved excision of the prosthesis, thorough soft tissue debridement and insertion of non-articulating antibiotic impregnated (vancomycin 2 g plus tobramycin 2.5 g in 40 g of bone cement) cement spacer [Table/Fig-1]. Intraoperatively, acute inflammation was present in periprosthetic tissue and multiple bony cysts were seen in both femur and tibia.
X-ray Left Knee in Anteroposterior (a) and Lateral (b) view showing the antibiotic cement spacer in situ following the first stage revision surgery for peri-prosthetic joint infection.

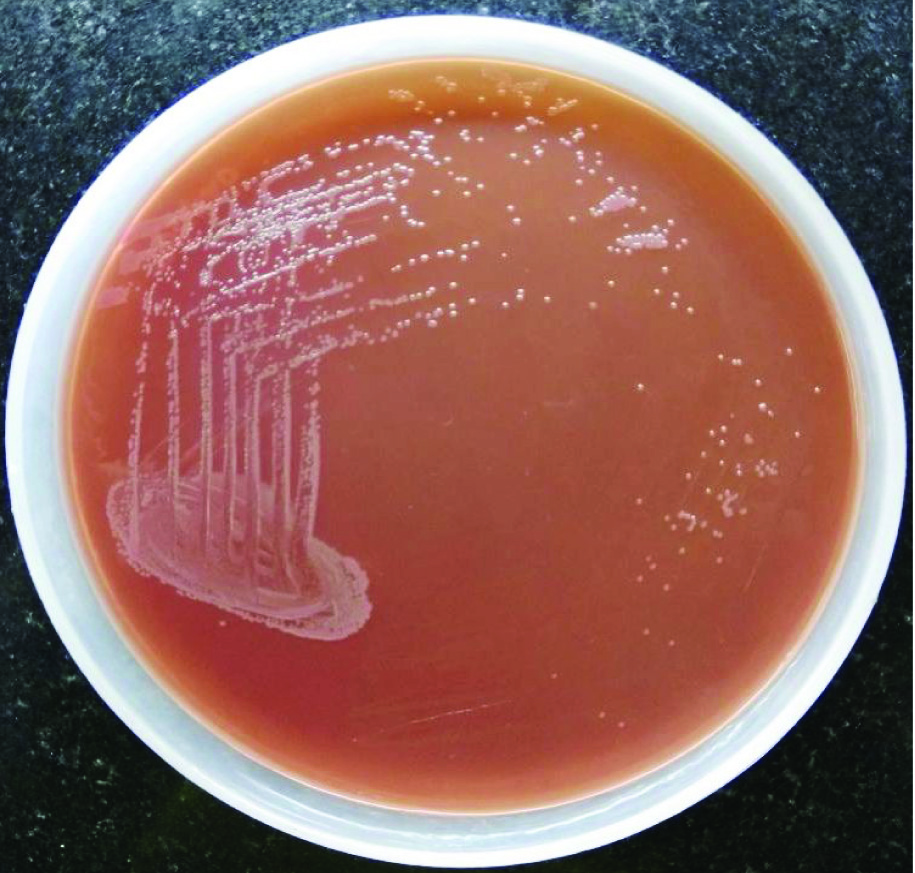
Sonication of the explanted implant was done by Ultrasonic bath (Bactosonic 14.2; Bandelin GmbH, Berlin, Germany). Aerobic cultures were performed on five periprosthetic tissue samples and prosthesis sonicate fluid. All six specimens were inoculated on 5% sheep blood agar and MacConkey agar for 48 hours and incubated aerobically at 37°C. After 48 hours of incubation culture yielded a pure growth of non-lactose fermenting colonies, mucoid, smooth colonies with diameter of approximately 2 mm [Table/Fig-2]. The organisms were identified as B. cenocepacia by MALDI-TOF MS system (Vitek MSTM; BioMe’rieux, France) and its confidence value of identification was 99.9%. Based on the antibiotic susceptibility testing [1], the organisms were susceptible to ciprofloxacin, meropenem, minocycline and co-trimoxazole but resistant to ceftazidime. The patient was treated with oral co-trimoxazole 500 mg for 12 hourly and ciprofloxacin 750 mg for 24 hourly.
Environmental samples including hospital water supply, cleaning and disinfectant solutions, intravenous fluids, swabs from the dressing trolley, bed railing, mattress and personnel attending the patient were collected to determine the source of infection. All the samples were culture negative for B. cenocepacia.
Following two month treatment, at outpatient follow-up, the patient reported no sign of infection and laboratory testing showed normalization of inflammatory markers. A repeat aspiration of the left knee was done two-months later and the synovial fluid culture was sterile. Subsequently, he was posted for the two-stage revision surgery. Bacterial culture of perioperative samples and sonicate fluid of extracted cement spacer obtained at the time of reimplantation were sterile. Post reimplantation, he was treated with co-trimoxazole and ciprofloxacin for four weeks. After a follow-up of eight months, the patient had no signs of recurrence of infection both clinically and radiologically [Table/Fig-3].
Non lactose fermenting, mucoid, smooth colonies of Burkholderia cenocepacia on MacConkey agar plate, after 48 hrs of incubation at 37°C in aerobic condition.
X-ray Left Knee in Anteroposterior (a) and Lateral (b) revision total knee prosthesis in situ following the second stage revision surgery.

Discussion
Members of Burkholderia cepacia complex (BCC) are taxonomically complex and comprise nine different genomovars [2]. The ability for asymptomatic bacterial colonization and potential for severe infections varies between different genomovars of BCC [3,4]. The clinical spectrum of BCC infection ranges from asymptomatic colonization to necrotizing granulomatous pneumonia in cystic fibrosis patients and bacteremia and pneumonia among hospitalized patients. Although all genomovars of BCC have been associated with human infections, B. cenocepacia (genomovar III) is one of the most common and most virulent genomovars [3,5]. B. cenocepacia is extremely resistant towards antibiotics and therapy is complicated by its ability to form biofilms [6].
The ability of B. cenocepacia to cause PJI is unusual. To the best of our knowledge, this is the first report of PJI caused by B. cenocepacia in an immunocompetent patient without cystic fibrosis. Several lines of evidence suggest that B. cenocepacia isolated from this patient was pathogenic and was responsible for PJI: (1) definite evidence of infection was present; (2) B. cenocepacia was isolated from all six intraoperative specimens; (3) there was an absence of other pathogens and (4) the infection responded to treatment.
Environmental sampling was done to trace the source of infection. However, the source of infection could not be concluded. We speculate that our patient acquired infection at the time of his first revision surgery due to contamination of the healthcare setting to which he was exposed. Evidence to support the ability of certain BCC strains to contaminate healthcare settings and to spread nosocomially has been provided by Mann T et al., [7]. The multitude of virulence factors possessed by this organism viz., biofilm formation, quorum sensing, outer membrane lipopolysaccharides etc., might have played a role in its ability to cause PJI. Patient’s co-morbidities may have also contributed to the establishment of the infection.
Members of the genus Burkholderia are innately resistant to various classes of antimicrobials. Owing to the high frequency of treatment failure and infection relapse, monotherapy is not recommended in BCC infections. Co-trimoxazole, ceftazidime and meropenem are active against B. cenocepacia [8]. In addition, ceftazidime and ciprofloxacin retain their antimicrobial activity against organisms embedded in biofilms. Since our isolate was resistant to ceftazidime, co-trimoxazole in combination with ciprofloxacin was given to our patient.
Accurate identification of the genomovars of BCC is of crucial importance to provide appropriate treatment with antibiotics. However, BCC identification to the species level by conventional microbiological techniques are challenging due to the phenotypic and genetic similarities among species [9]. Therefore, reliable and accurate identification of BCC requires the use of newer molecular techniques. MALDI-TOF has been shown to be a reliable and rapid technique for accurate bacterial identification. In a recent study done by Peel NT et al., MALDI-TOF MS was found to be a valuable diagnostic tool for the early identification of isolates from PJI patients [10].
Conclusion
The association of B. cenocepacia with PJI further extends the clinical spectrum of this opportunistic pathogen. Though high mortality and morbidity are the hallmarks of B. cenocepacia infections, perioperative vigilance, timely submission of properly obtained cultures, rapid identification of the pathogen by MALDI-TOF MS made it possible to recognize the infection and treat it with appropriate antibiotics.
[1]. Clinical Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing. Twenty sixth informational supplement M100-S26. CLSI, Wayne, Pennsylvania, USA, 2016 [Google Scholar]
[2]. Vermis K, Coenye T, Mahenthiralingam E, Nelis HJ, Vandamme P, Evaluation of species-specific recA-based PCR tests for genomovar level identification within the Burkholderia cepacia complexJ Med Microbiol 2002 51:937-40. [Google Scholar]
[3]. Drevinek P, Mahenthiralingam E, Burkholderia cenocepacia in cystic fibrosis: epidemiology and molecular mechanisms of virulenceClin Microbiol Infect 2010 16(7):821-30. [Google Scholar]
[4]. Woods CW, Bressler AM, LiPuma JJ, Alexander BD, Clements DA, Weber DJ, Virulence associated with outbreak-related strains of Burkholderia cepacia complex among a cohort of patients with bacteremiaClin Infect Dis 2004 38:1243-50. [Google Scholar]
[5]. LiPuma JJ, Spilker T, Gill LH, Campbell PW, Liu L, Mahenthiralingam E, Disproportionate distribution of Burkholderia cepacia complex species and transmissibility markers in cystic fibrosisAm J Respi Crit Care Med 2001 164:92-96. [Google Scholar]
[6]. Caraher E, Reynolds G, Murphy P, McClean S, Callaghan M, Comparison of antibiotic susceptibility of Burkholderia cepacia complex organisms when grown planktonically or as biofilm in vitroEur J Clin Microbiol Infect Dis 2007 26(3):213-16. [Google Scholar]
[7]. Mann T, Ben-David D, Zlotkin A, Shachar D, Keller N, Toren A, An outbreak of Burkholderia cenocepacia bacteremia in immunocompromised oncology patientsInfection 2010 38(3):187-94. [Google Scholar]
[8]. Avgeri SG, Matthaiou DK, Dimopoulos G, Grammatikos AP, Falagas ME, Therapeutic options for Burkholderia cepacia infections beyond co-trimoxazole: a systematic review of the clinical evidenceInt J Antimicrob Agents 2009 33(5):394-404. [Google Scholar]
[9]. McMenamin JD, Zaccone TM, Coenye T, Vandamme P, LiPuma JJ, Mis-identification of Burkholderia cepacia in U.S. cystic fibrosis treatment centers: an analysis of 1051 recent sputum isolatesChest 2000 117(6):1661-65. [Google Scholar]
[10]. Peel NT, Cole CN, Dylla LB, Patel R, Matrix-assited laser desorption ionization time of flight mass spectrometry and diagnostic potential for prosthetic joint infection in the clinical microbiology laboratoryDiag Micro Infec Dis 2015 81:163-68. [Google Scholar]