Dental magnets have been widely used in Prosthodontics. Magnetic attachments have most commonly been used for the retention of mandibular overdentures. Patients with magnet retained overdentures have reported a high degree of satisfaction with their dentures [1].
Neodymium-Iron-Boron (Nd-Fe-B) and cobalt samarium magnets have been widely used in dentistry [2]. To improve the retention of the mandibular denture often few teeth or root stumps were retained in the patients mouth, which were further used to embed a dental magnetic attachment [3]. Most of the times corrosion by oral fluid was the major disadvantage of these magnets [4-6]. Both the cobalt samarium and the neodymium iron boron are extremely brittle and susceptible to corrosion, especially in chloride containing environments such as saliva. The corrosion products from rare earth magnets also have been shown to have cytotoxic effects in in-vitro tests [7,8].
Ensuring biological safety of any newly developed biomaterial is of prime importance before it is being put into clinical use. The material or implantable device should not cause any side effects to the tissues either locally or at systemic level. Biological testing of dental magnets has been done previously with respect to the effect of static magnetic field and possible toxic effects of the material or their corrosion products on the surrounding tissues. Biological testing lies greatly on animal experimentation. Before a dental material can be used clinically, it is mandatory to test the material to the fullest extent in species of laboratory animals to establish its systemic and cytotoxic properties [9]. The use of animals assists to foresee the possible toxic vulnerabilities that may be confronted in man.
The corrosion products released from the magnets may cause possible toxic effects. Therefore, the aim of this research was to investigate the in-vivo cytotoxic effect of the indigenously fabricated dental magnet in a rabbit animal model and to compare the tissue response between non-encased magnet and encased magnet.
Materials and Methods
Fabrication of Magnet: The present study was undertaken at SDM College of Dental Sciences and Hospital, Dharwad, Karnataka, India. The total duration of the study was three years which included step-wise in-vivo evaluation of the fabricated magnet. Fabrication of magnet was done in six months which was done in collaboration with Magnatech engineering Mumbai, India. A rare earth magnet Neodymium-iron-boron (Nd-Fe-B) was machine cut to get the desired dimension. The magnet was in a circular shape. The diameter of the magnet was designed based on the average value of cross-sectional diameter of crown portion of the mandibular canine and premolar tooth. The diameter of the Nd-Fe-B magnet was 3 mm and 1.5 mm thick in height. The basic design of the magnet was pot-type. Pot-type means that the magnet was encased in the corrosion resistant material. Bare magnets readily corrode in the oral environment because of acidic nature. Therefore a coating or casing is mandatory to prevent corrosion of the magnet [10]. Teflon was used to encase the bare magnet to act as corrosion resistant material.
Procedure: The study was conducted on five New Zealand white rabbits. All the rabbits were between 1 to 1.5 years of age and weighed between 2.5 to 3 kg. The animals were housed in the cage at the animal house, Department of Pharmacology, SDM Medical College and Hospital, Dharwad. Instituitonal Ethical Committee approval was taken prior to conducting the study. The animal house was recognized by the guidelines of Committee for the purpose of Control and Supervision of Experiments on Animals in India.
The surgery was done under the supervision and guidance of pharmacologist and veterinary doctor. A total of 10 magnets were implanted subcutaneously in the rabbits on either side of vertebra (back of rabbit). Among the 10 magnets used 5 were bare non-encased magnets (Magnatech Engineering Co; Mumbai, MS, India) which formed the controls and other 5 were indegenously fabricated teflon encased dental magnets (test).
The animals were anaesthetized with phenobarbitone at a dose of 54 mg/kg body weight. [11] Once they got anaesthetized surgical procedure was carried out. Hair around the surgical site was removed with scissors. A small sub cutaneous incision was made on the back region of the rabbit on right and left side with a no. 15 BP blade. The skin layers were slightly dissected under the incision creating a pouch. The non-encased bare Nd-Fe-B magnet (control) was placed on the right side incision pouch and sutured. Similarly the incision was placed on the left side and the pouch was made. Here the teflon encased (test) magnet was placed and sutured. A 3-0 synthetic, braided, absorbable suture was used (Vicryl; Ehticon, Johnson and Jhonson, Aurangabad, India).
Post-operatively, the animals were provided with their regular food. No drugs were administered, except for topical application of antiseptic cream on the surgical site. All the rabbits recovered from surgery.
After the complete healing in 28 days, standardized punch biopsies were taken along with the implanted magnet from the first three rabbits. For the 4th and 5th rabbit biopsy was done after three months. The raw area was allowed to heal and closed dressing was placed at the biopsy site. In the present study the collected implanted magnet was observed macroscopically for its relation to the surrounding tissue.
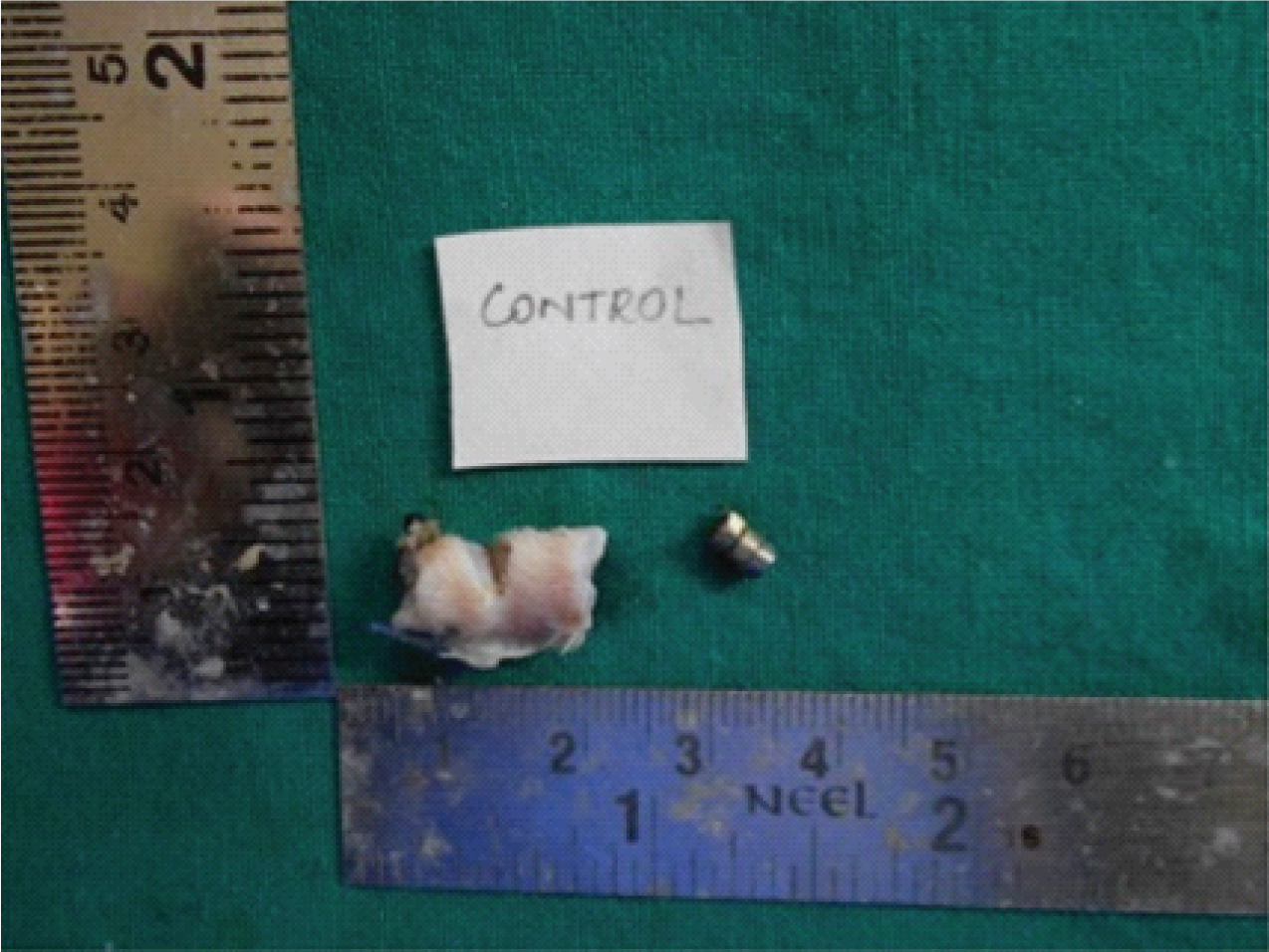
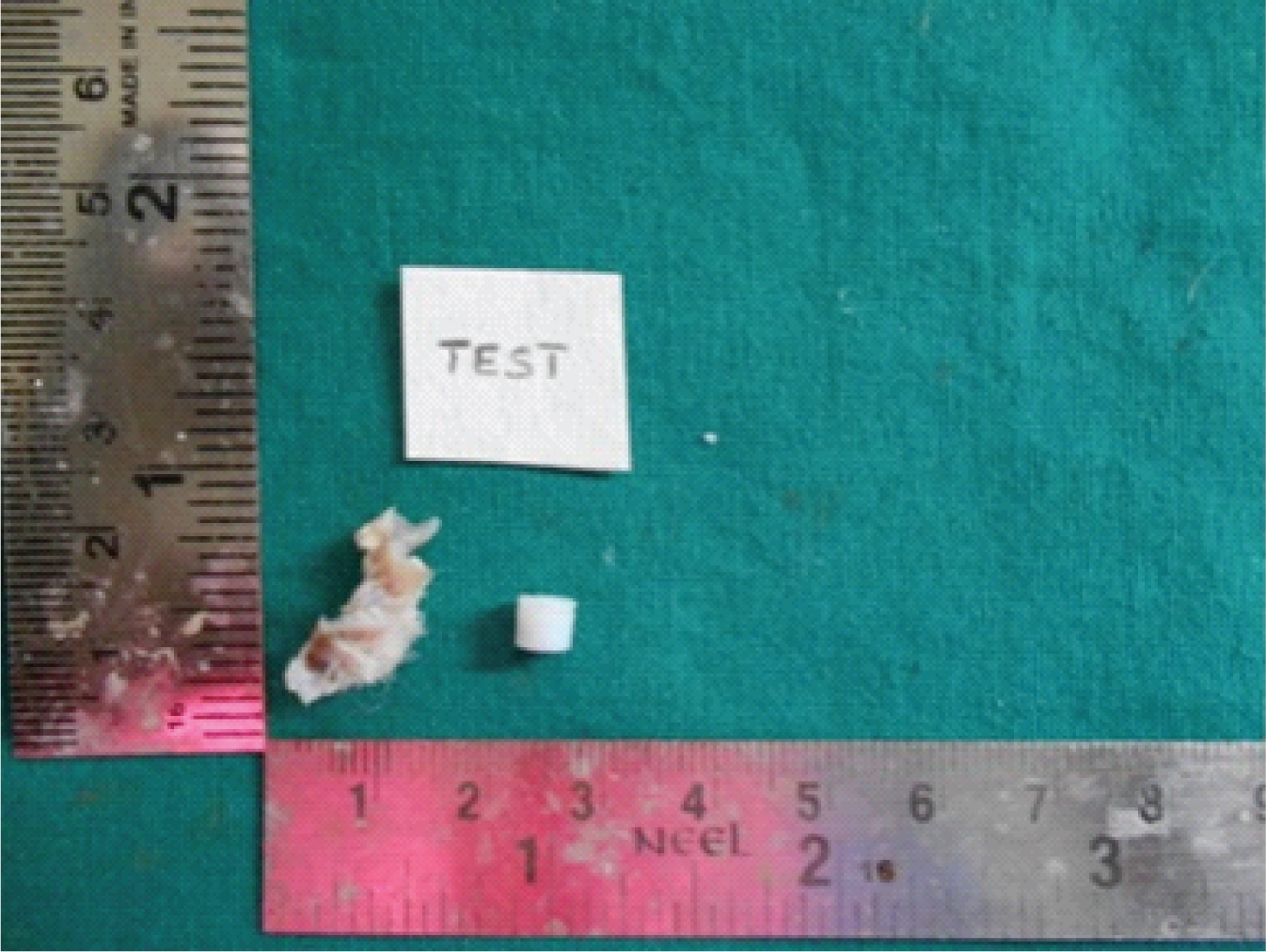
Biopsy samples were obtained at both the site of magnets placed in control and test. The samples were further sub-divided into two halves in the center [Table/Fig-1,2]. The cut surface of the specimen showed epidermis and dermis, colour of the tissue is creamish to white in colour. Following the gross examination tissues were fixed in 10% neutral buffered formalin for 5 days, processed and embedded in paraffin wax. Sections of 4 μm thickness were cut using semiautomatic microtome and sections were stained with Haematoxylin and Eosin (H&E).
Macroscopic appearance of control specimen oval in shape creamish white in colour with hair on the surface.
Macroscopic appearance, creamish to light brownish in colour with hair.
H&E stained sections were evaluated for histological changes inclu-ding the inflammatory response both acute and chronic inflammatory cells, multinucleated giant cells and stroma fibrous or loosely arranged collagen fibers with fibroblast cells, neovascularization and foreign body reaction status epithelial thickness and keratinization with respect to time using a binocular optical microscope. The level of inflammatory reaction to an implanted magnet is used to assess the biocompatibility of the magnet. Data was collected based on the score set for different observations. The scores were given for different observations of epidermis. For thickness of epithelium: 1-thin, 0- normal; for keratinization: 0- mild, 1- moderate; for dermal fibres: 0-single, 1-dense and for inflammation: 0-mild, 1- moderate. In the present study, severe inflammation was not observed. Hence, the scoring was given only for mild and moderate inflammation. Non-parametric Mann-Whitney U test was done to assess the statistical significance. Alpha was set at p<0.05.
Results
The results for the histological features of tissues after 28 days are presented in [Table/Fig-3]. There was no change in the epidermis thickness and dermal inflammation in either group after 28 days, whereas a statistically significant difference in epidermis keratinization and dermis fibres arrangement was seen with majority having moderate keratinization and dense dermis arrangement in test group. The results of the test after three months are presented in [Table/Fig-4]. After three months there was a statistically significant difference in the epidermis thickness with test group having normal epidermis. The statistically significant difference seen in keratinization at 28 days was still observed after three months. There was no difference in dermal fibres arrangement or chronic inflammation at the end of three months between the two groups.
The histological features of tissues after 28 days.
| Varialbes | Epidermis | Dermis |
|---|
| Thickness | Keratinized | Fibres | Inflammation |
|---|
| Median histological features of tissues in test group | 1 | 1 | 1 | 0 |
| Median histological features of tissues in control group | 1 | 0 | 0 | 0 |
| Mann-Whitney U | 12.500 | 0.000 | 5.000 | 7.500 |
| p-value | 1.000 | 0.003* | 0.050* | 0.134 |
The histological features after 3 months.
| Varialbes | Epidermis | Dermis Chronic |
|---|
| Thickness | Keratinized | Fibres | Inflammation |
|---|
| Median histological features of tissues in test group | 0 | 1 | 1 | 0 |
| Median histological features of tissues in control group | 1 | 0 | 1 | 0 |
| Mann-Whitney U | 5.000 | 2.500 | 10.000 | 7.500 |
| p-value | 0.050* | 0.014* | 0.317 | 0.134 |
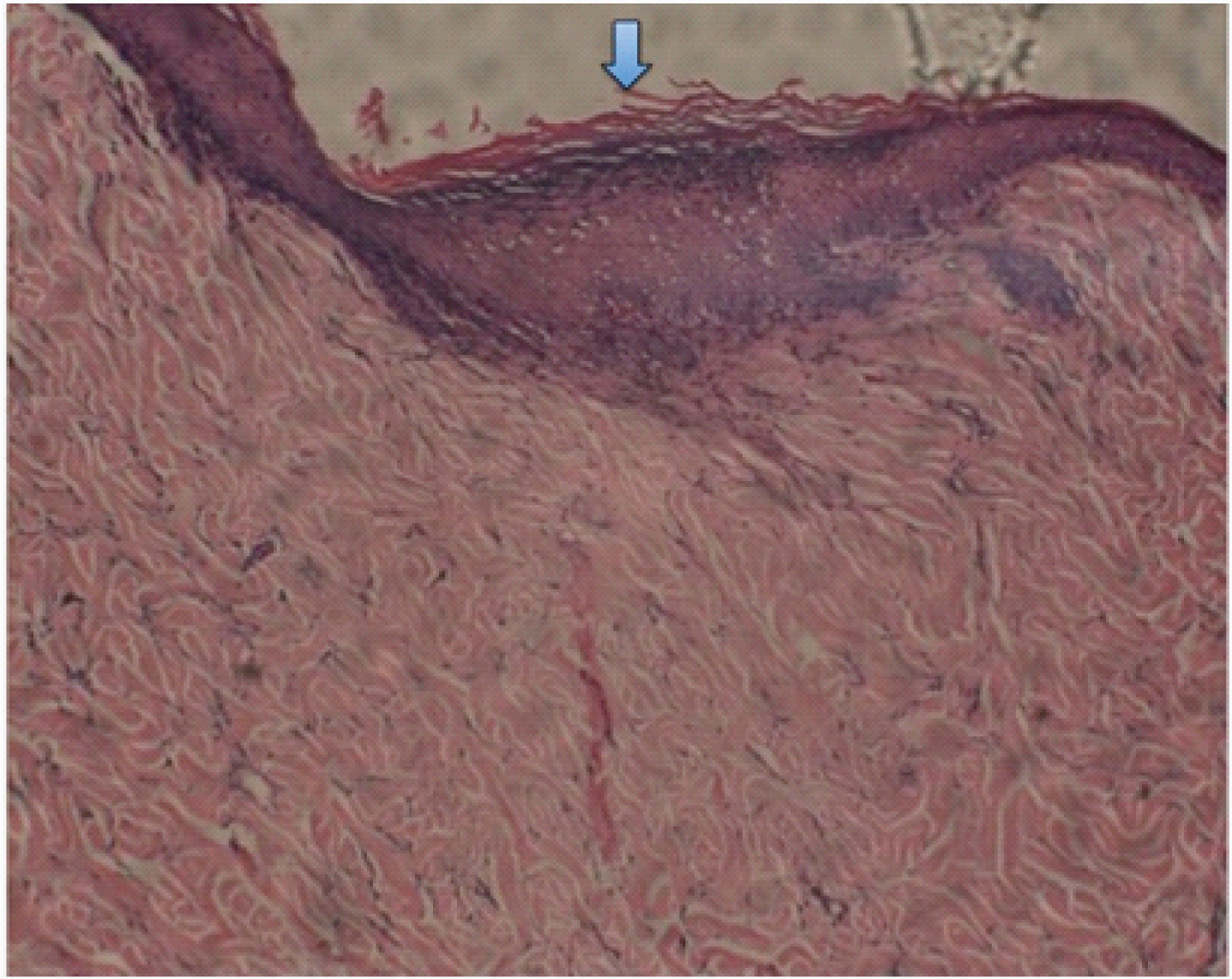
Microscopic features show epidermis and the underlying dermis. The epidermis showed thin and composed of 3-5 layers of epithelium with the papillary projections in areas. Dermis consisted of hair follicles (inner and external root sheath), few blood vessels and sparse, dispersed chronic inflammatory cell infiltrate in a densely arranged fibrous stroma.
The control and test samples at 28 days [Table/Fig-5,6 and 7] revealed keratinized epidermis with minimal/mild chronic inflammatory cell infiltrated in the fibrous dermis. The dermal appendages were normal. The control samples at 3 months [Table/Fig-8,9] displayed keratinized epidermis with normal number of dermal appendages in the fibrous dermis. The test samples at 3 months [Table/Fig-10] showed similar features with increased epithelial thickness and keratinization; and with appendages at the dermis are replaced by fibrosis.
Control specimen at 28 days. 4X Photomicrograph shows thin keratinized epidermis with dermal appeandages. Stroma is less fibrous with few muscle fibers at the deeper layer.
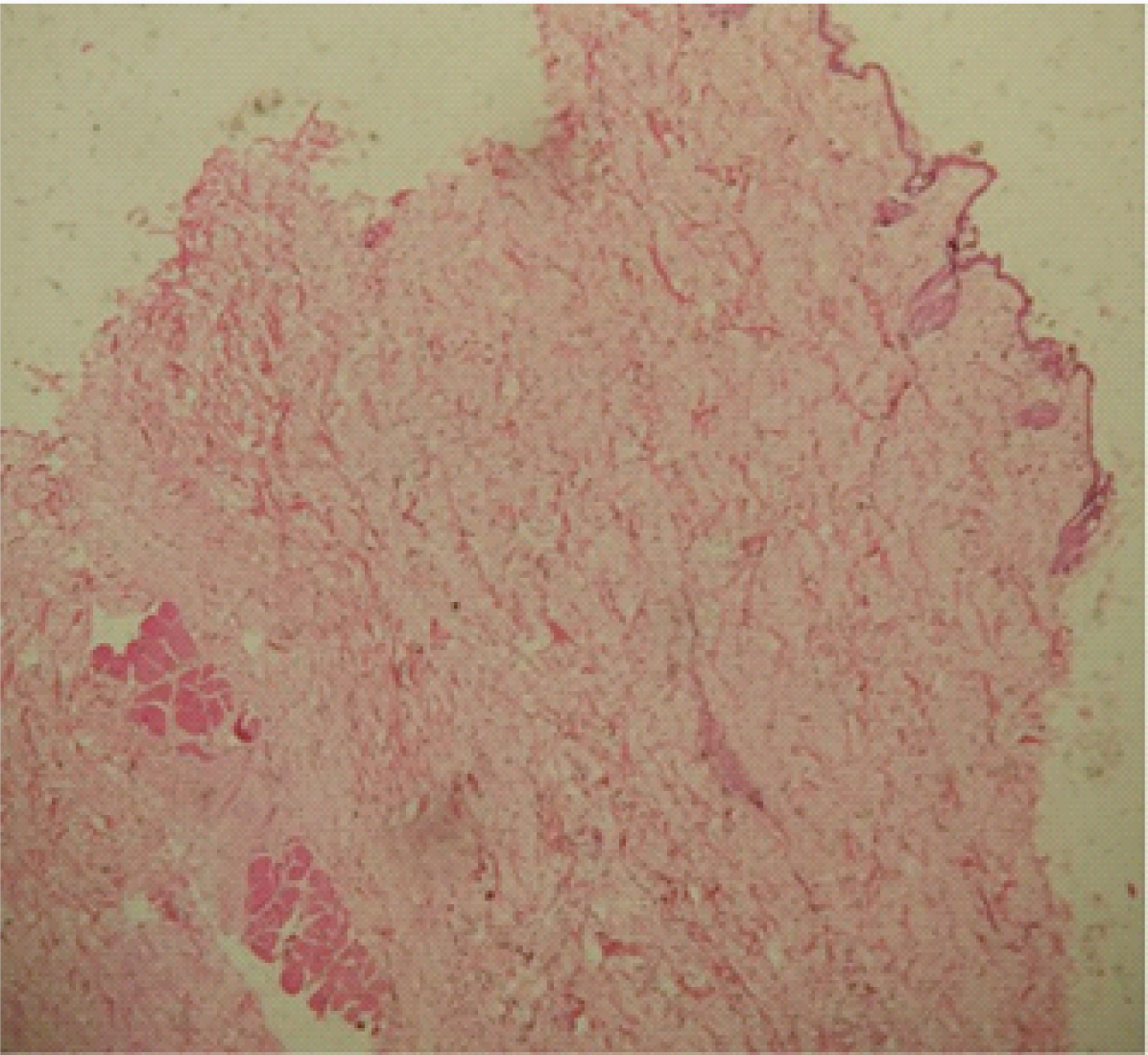
Control specimen at 28 days. 10X Photomicrograph shows thin keratinized epidermis with dermal appandages in the Stroma.
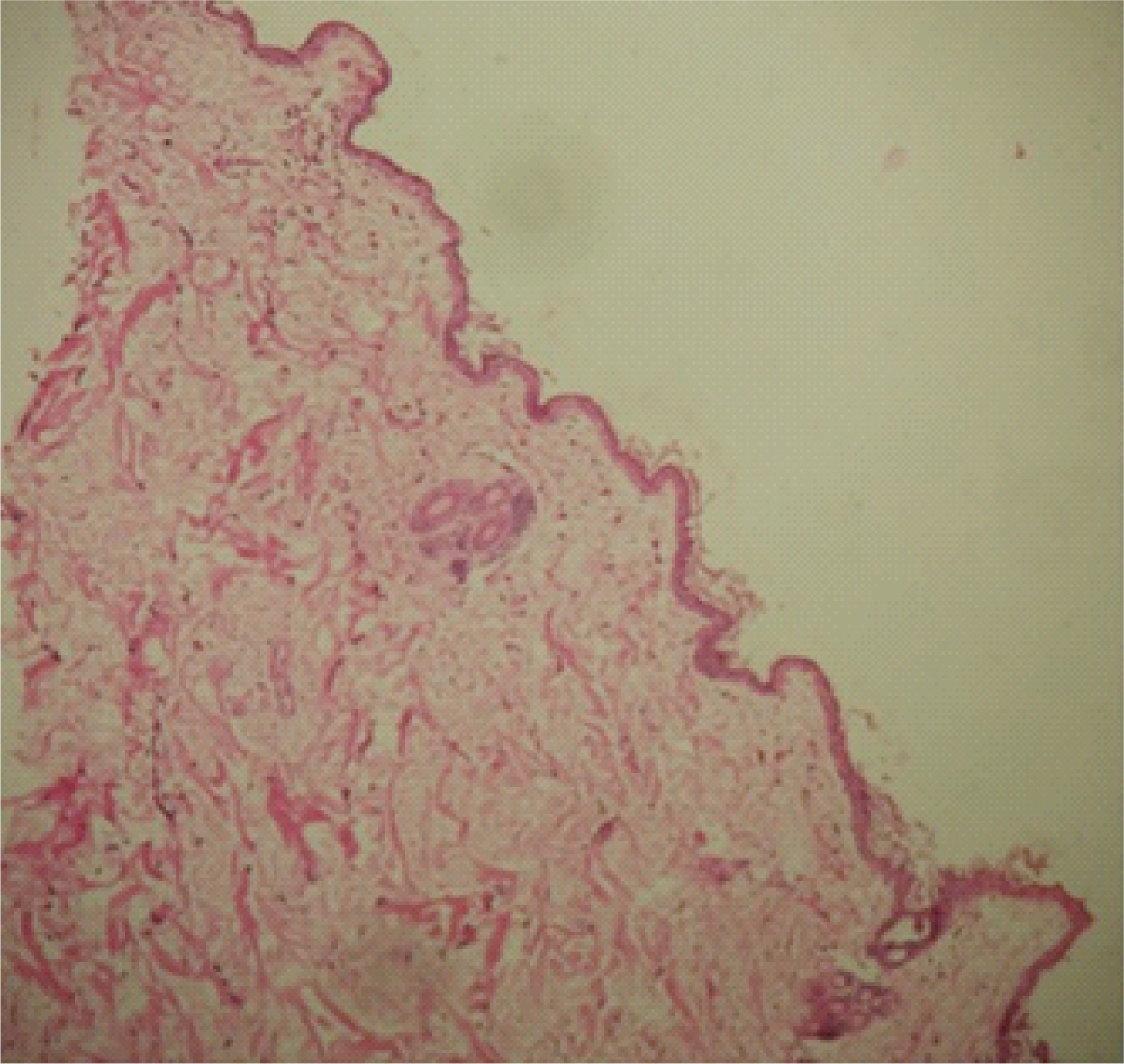
Test specimen at 28 days. 4X photo micrograph shows keratinized evenly thickened epidermis with dermal appeandages and minimal lymphocytes in the sub-epidermal area. Muscle layer seen in deeper zone.
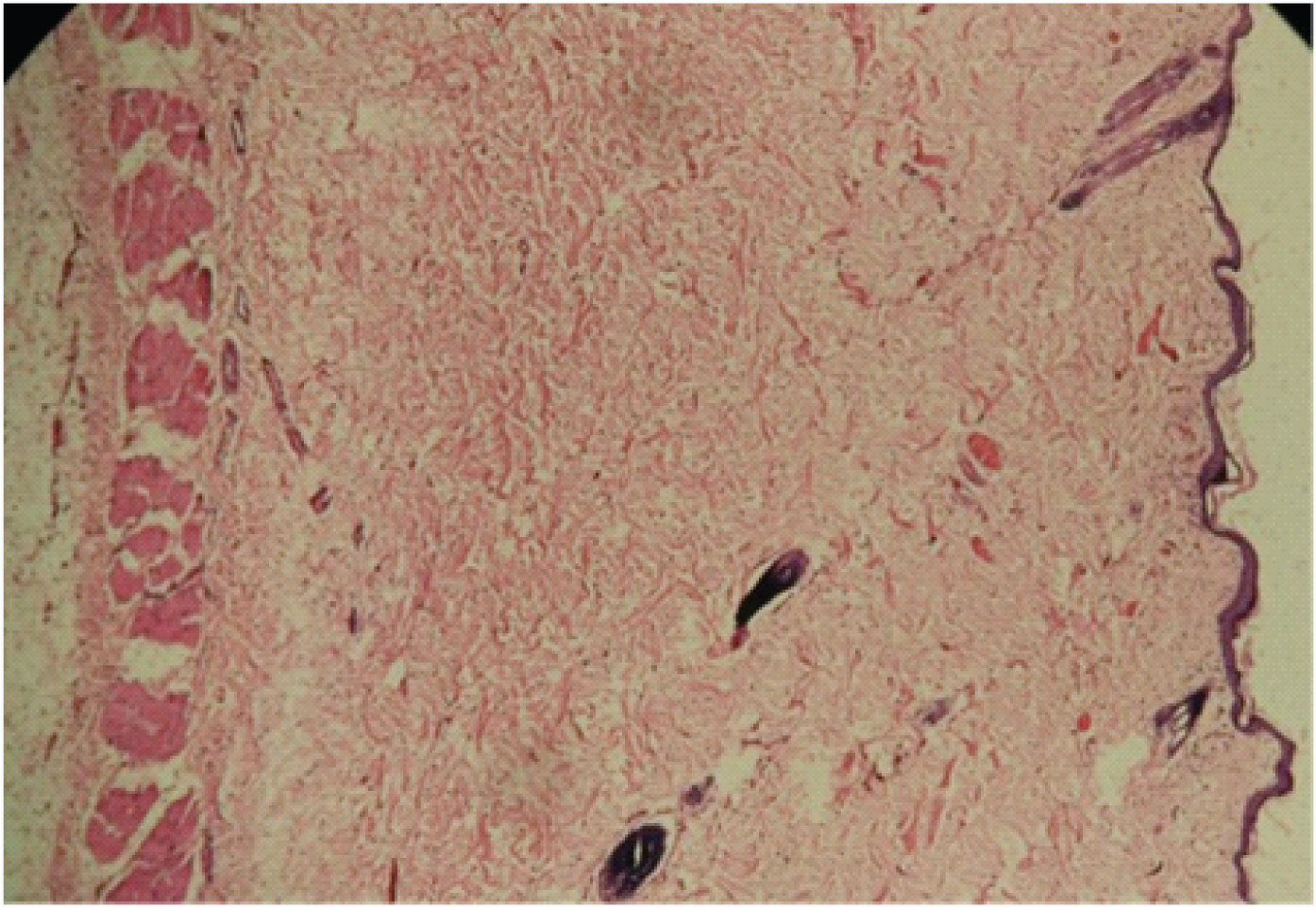
Control at 3 months. Shows mild keratinized epithelium with mild variation in thickness. Stroma is fibrous with short bundles of collagen fibers. Numerous dermal appendages and focal lymphocytic cell infiltration (4X).
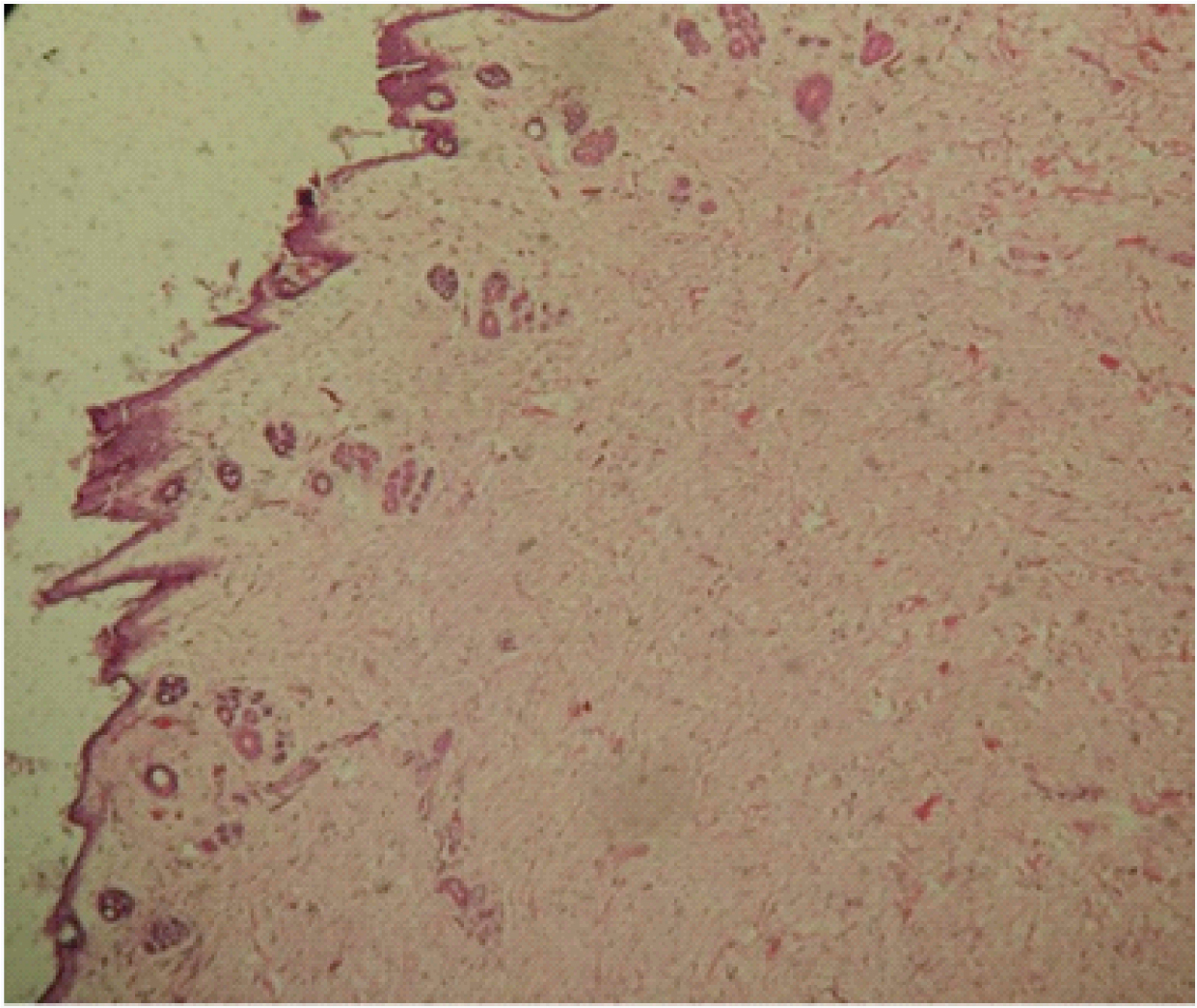
Control at 3 months with high magnification 4x.
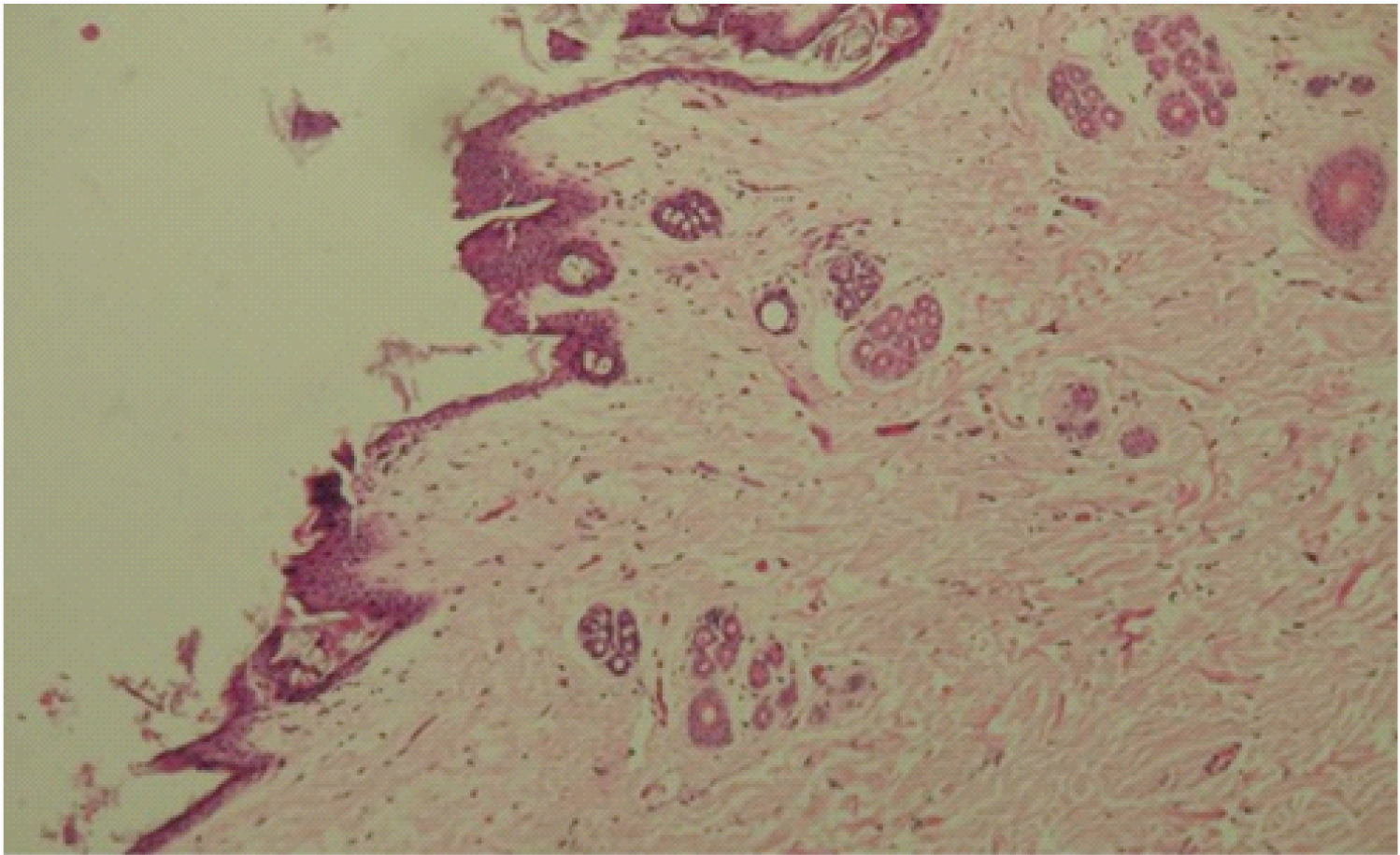
Test specimen at 3 months. Keratinized epithelium with increased thickness (arrow) and dermis fibrosis.
Discussion
The aim of the study was to check the in-vivo tissue reaction of indigenously fabricated dental magnet implanted in an animal model. The magnet used was Nd-Fe-B and encased with Teflon. Basically, the magnets have cytotoxic potential in oral environment due to their corrosive nature. Corrosion of dental materials and other implantable materials like metals poses a serious problem for their usage in clinical scenarios.
Materials used in various dental applications are exposed to mechanical loading and pH causing rapid corrosion of metallic materials [12]. Metal ions are released from dental materials in-vitro and in-vivo [13,14]. Released metal ions in dental and other applications can cause staining of the surrounding tissue, mild to severe local inflammation up to systemic effects, such as sensitivity and allergic reactions [15,16].
Host tissue reactions secondary to the magnets include, initially injury, damage to local blood vessels later chronic inflammatory and foreign body reaction with granulation tissue formation. During the healing phase, granulation tissue is replaced by fibrosis [17]. Histopathological examination of acute tissue reaction reveals presence of neutrophils and macrophages. Acute inflammation usually subsides within a week. But, if the irritant/foreign substance in the tissue is not removed, the inflammation transforms to chronic condition. The neutrophils decrease in number and these are replaced by lymphocytes, plasma cells and macrophages. Foreign body multinucleated giant cells can be formed by the fusion of macrophages adjacent to the foreign particle [17].
To reduce the host reaction the indigenously fabricated dental magnet was encased in a teflon cylinder. The Teflon cylinder housed the Nd-Fe-B magnet and it was sealed with a Teflon lid using cyanoacrylate resin. Bondemark L et al., conducted cytotoxic study on samarium-cobalt and NdFeB magnets [7]. They showed that the polymeric based material coating on these magnets like parylene and Teflon had negligible cytotoxicity. Short-term exposure to a static magnetic field did not cause any cytotoxic effect on the cells. In another study conducted by Bondemark L et al., on effect of magnets on buccal mucosa, found that there was minor tissue reaction that were construed as a result of micro trauma and not caused by static magnetic field per se, since there was no difference between test and control tissues [18].
There are contradicting results in the literature when it comes to the toxic effects of dental magnet on the tissues. Few researchers showed harmful biological effects on different mammalian tissues. [19-22] Haley TJ [23], in his study of pharmacology and toxicology involving rare earth magnets showed low acute toxicities. However, other investigators like Behrman SJ [24] reported successful results from his study wherein Pt-Co magnets were implanted in mandibles. Toto PD et al., studied the reaction of bone to the Pt-Co magnetic implant in dogs and observed no foreign body reaction [25]. Cerny examined the reactions of dental tissues to the magnetic field of Sm-Co magnets which can be used in orthodontics or prosthodontics no histological alteration was observed in the tissues for 6 months [26]. In another study, Cerny R et al., examined the effects of samarium-cobalt (Sm-Co) magnets in mammalian tissues and blood cells and no harmful effects were observed [27,28].
The tissue compatibility of the implanted magnet depends upon the response of the cells on the material and vice-versa. One of the most important factors that affect the material in-vivo biocompatibility is the cellular interaction, which occurs at the material-tissue interface. Histopathological techniques have been widely adopted to determine the tissue tolerance of material with subcutaneous implanted magnet and intramuscular implanted magnets [24].
During different periods of implantation, it is difficult to analyse the material/cell interactions at the interface, under in-vivo conditions with the histopathological techniques. Therefore in the present study it was decided to observe tissue reaction to a magnet at three intervals one at 28 days and at three months duration. The reason is at the end of 28 days the wound would be stabilized and acute phase will be transformed to chronic phase. It has been observed that the neutrophils stop migrating into site after eight hours in rabbits [29]. Moreover, the neutrophils in tissues have shorter life and die at the site of inflammation [29].
Acute reaction in tissue is characterized by the presence of polymorphonuclear leukocytes and resolve within one week. The inflammation becomes chronic and is represented by the presence of lymphocytes, plasma cells and macrophages, may or may not be associated with multinucleated giant cells and with the biocompatible material the chronic inflammation no longer lasts than two weeks.
Recently importance has been given to growth factors and inhibitors produced by mononuclear phagocytes and their role in the chronic inflammatory reaction [30]. These growth factors produced by monocytes and macrophages and other inflammatory cells have implications in the implanted magnet associated with wound healing responses [30].
In the present study, in the first three animals punch biopsies of control and test tissues were obtained at 28 days, histological features revealed only the presence of chronic inflammatory cells in the dermis with normal dermis and as mentioned above the acute phase had resolved. Whereas, the punch biopsies of test obtained at three months revealed minimal chronic inflammatory cells in the collagenous stroma and gradual increase in the thickness of the epithelium indicates response of the tissue maturation. These features favour the biocompatibility of Teflon encased magnet with tissue. The tissue reaction in the test and control samples was closely similar. The healing of tissue following 28 days was good. These results indicate the suitability and acceptability of indigenously fabricated dental magnet to be used clinically on humans.
Limitation
However, one of the main limitations of this study would be the small sample size. Often, fewer animals are used to make it possible to detect significant effect, as use of animals for experimental research becomes an ethical issue.
Conclusion
To conclude indigenously fabricated dental magnet did not show host inflammatory reaction in the animal study model over the control magnet. Histopathological evaluation revealed that there was minimal chronic inflammatory cells in the collagenous stroma, over the period of three months indicating a good healing of the surgical site and thus proving its biocompatibility. This study should be followed by similar supportive studies in humans.