Tooth Extraction in a Hepatic Cirrhosis Patient Receiving the Novel Oral Thrombopoietin Receptor Agonist Lusutrombopag
Shin-Ichiro Hiraoka1, Kohei Kawamura2, Tetsuya Seikai3, Tadataka Tsuji4, Mikihiko Kogo5
1 Assistant Professor, First Department of Oral and Maxillofacial Surgery, Graduate School of Dentistry, Osaka University, Suita, Osaka, Japan.
2 Graduate Student, First Department of Oral and Maxillofacial Surgery, Graduate School of Dentistry, Osaka University, Suita, Osaka, Japan.
3 Clinical Fellow, First Department of Oral and Maxillofacial Surgery, Graduate School of Dentistry, Osaka University, Suita, Osaka, Japan.
4 Clinical Fellow, First Department of Oral and Maxillofacial Surgery, Graduate School of Dentistry, Osaka University, Suita, Osaka, Japan.
5 The Chair, First Department of Oral and Maxillofacial Surgery, Graduate School of Dentistry, Osaka University, Suita, Osaka, Japan.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Shin-ichiro Hiraoka, Assistant Professor, First Department of Oral and Maxillofacial Surgery, Graduate School of Dentistry, Osaka University, 1-8 Yamada-Oka, Suita, Osaka, Japan.
E-mail: hirashins2@gmail.com
Lusutrombopag was administered to a patient with thrombocytopenia due to chronic liver disease before an invasive procedure. Lusutrombopag, the first drug of its kind, was developed to improve platelet count thereby, avoiding the need for platelet transfusion. Lusutrombopag is potentially effective for the management of haemostasis during invasive procedures, such as tooth extraction. Herein, we present a case of tooth extraction surgery in a patient treated with lusutrombopag. The patient was a 63-year-old woman suffering from thrombocytopenia due to liver cirrhosis. Tooth extraction was indicated by the diagnosis of caries and apical periodontitis of the maxillary left second molar. Mild spontaneous bleeding in the surrounding gum tissue and a reduced platelet count (5.1 × 104/μL) were observed. Haemostatic difficulties were predicted to accompany tooth extraction surgery. Consultation with a gastroenterology clinic led us to implement lusutrombopag prior to the procedure. The patient’s platelet count had risen to 10.9 × 104/μL by the day of the surgery. Neither intraoperative nor postoperative bleeding was observed. No adverse events associated with lusutrombopag administration were observed. Lusutrombopag can increase platelet count, suggesting its utility in invasive procedures such as tooth extraction.
Liver cirrhosis, Periodontitis, Platelet, Thrombocytopenia
Case Report
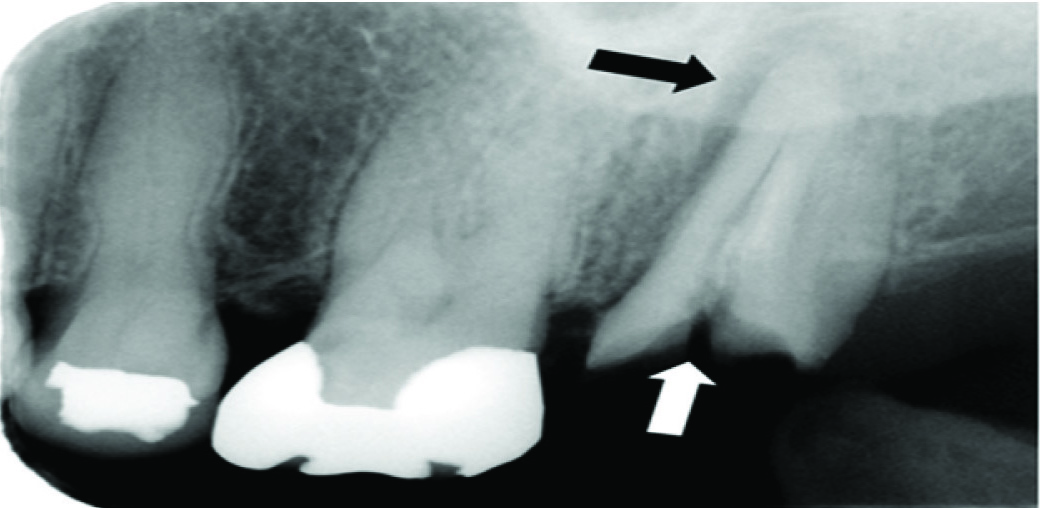
A 63-year-old was referred to our hospital in April 2016 after signs of severe infection and bleeding around her maxillary left second molar. She had developed cirrhosis-induced thrombocytopenia and splenomegaly following treatment for chronic Hepatitis C Virus (HCV) infection. In September 2015, she had received ledipasvir/sofosbuvir treatment at the Hepatology Department of another hospital. She subsequently tested negative in an HCV antibody test, but her platelet count did not increase. Her family history was unremarkable. The patient had a moderate build, good nutritional status and no evidence of systemic bleeding. She exhibited caries in the maxillary left second molar, swelling, redness and mild spontaneous bleeding in the surrounding gum tissue. Bone resorption was observed around the maxillary left second molar in X-ray radiography [Table/Fig-1].
Radiograph of the maxillary left molar region at the initial visit. Root fracture due to caries is visible (white arrow). Increased X-Ray penetration in the apical region, as well as periodontal space widening, attributable to periodontitis (black arrow), can be seen.
Clinical laboratory tests revealed normal white blood cell (3.5 × 103/μL) and red blood cell counts (4.29 × 106/μL), but a low platelet count (5.1 × 104/μL) [Table/Fig-2].
Treatment course and corresponding platelet counts.
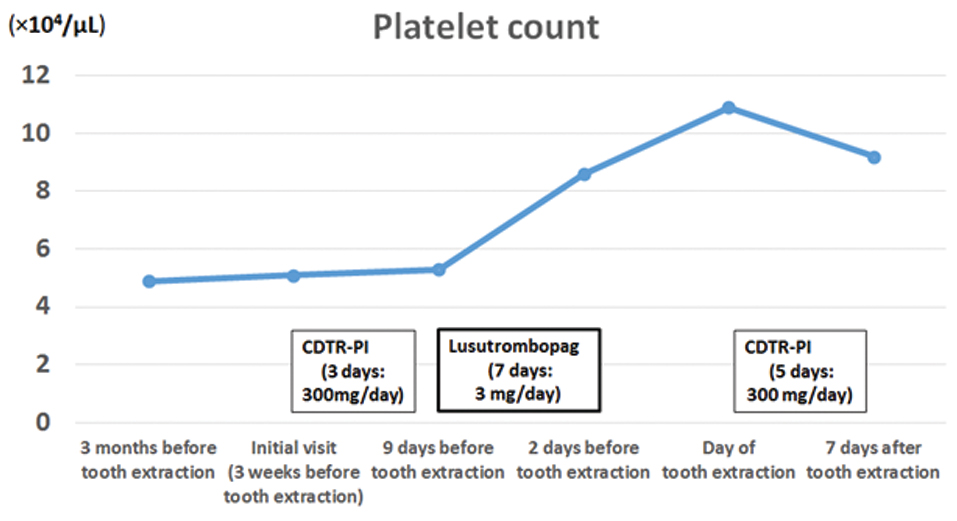
Prothrombin Time (PT) and PT-international normalized ratio were somewhat elevated (12.5 s and 1.16, respectively). Aspartate aminotransferase, alanine aminotransferase, gamma-glutamyl transferase and C-reactive protein levels were 40 IU/L, 34 IU/L, 40 IU/L and 0.1 mg/dL, respectively. Quantitative HCV RNA tests were negative. The patient was diagnosed with caries and apical periodontitis of the maxillary left second molar.
Because of the localized inflammation, we prioritized antiinflammatory treatment before tooth extraction. She received cefditoren pivoxil (CDTR-PI: 300 mg/day) for 3 days, along with local irrigation. According to the gastroenterology clinic, where she was receiving followup for her HCV treatment, she had exhibited repeated episodes of spontaneous gum bleeding and periodic nosebleeds. Therefore, we decided to administer lusutrombopag to prevent bleeding after tooth extraction. The patient was started on a 7-day course of lusutrombopag (3 mg/day), prior to extraction surgery. Her platelet count rose to 10.9 × 104/μL by the day of surgery; we then performed left maxillary second molar extraction under local anaesthesia and sutured the wound with silk thread. No significant bleeding was observed during or after surgery. CDTR-PI was administered for 5 days post-surgery (300 mg/day). Her platelet count remained high (9.2 × 104/μL) 7 days after tooth extraction [Table/Fig-2]. Wound healing was uneventful thereafter.
Discussion
Platelet counts are reduced in many patients with chronic liver disease due to hypersplenism and reduced thrombopoietin (TPO) production [1-3]. Thrombocytopenia increases the bleeding risk during invasive procedures [2], which may cause a delay or even discontinuation of a patient’s treatment [3].
When chronic liver disease patients require tooth extraction or other oral surgical procedures, dentists and surgeons may consider performing platelet transfusion as an adjunctive treatment to reduce postoperative bleeding risk. However, this poses risks of infection and other complications [4]. Accordingly, there has been a demand for a safe drug that can reliably and effectively increase platelet count. One such drug, eltrombopag, has now been approved for use in idiopathic thrombocytopenic purpura [5]. Lusutrombopag is a TPO receptor that was developed to negate the need for platelet transfusion prior to invasive procedures in patients with thrombocytopenia caused by chronic liver disease, by improving platelet count. It is the first drug of its kind [6] and has shown promising clinical results [7], but its use for managing haemostasis during tooth extraction in a patient with thrombocytopenia due to liver cirrhosis, such as we described here, has not been reported to date.
Lusutrombopag promotes the phosphorylation of Janus kinase 2, signal transducer and activator of transcription 3, and other signal transducers and activators of transcription, as well as p44/42 mitogen-activated protein kinase. Thus, it is believed to increase platelet count by selectively acting as an agonist of human TPO receptors activating certain TPO signalling pathways, which in turn induces human bone marrow progenitor cells to proliferate and differentiate into megakaryocytic lines [8,9].
Lusutrombopag demonstrated excellent efficacy in a phase III clinical trial in which the primary outcome was the platelet transfusion avoidance rate (placebo group 12.5%, lusutrombopag group 79.2%) [7]. It received regulatory approval in Japan in September 2015, which was the first approval for its use globally [6]. Its recommended dosage is a 3 mg dose per day and treatment should begin 8-13 days prior to an invasive procedure [8].
The longterm safety of this drug is yet to be established in terms of coincidence of thrombotic and other events and medical professionals must be watchful for any future reports on its side effects. In the aforementioned phase III clinical trial, the number of adverse events between the lusutrombopag group and placebo group did not differ significantly [7]. However, serious thrombotic events were observed in 2% of patients who received lusutrombopag (e.g., mesenteric vein thrombosis, portal vein thrombosis) [8], suggesting that thrombosis occurrence is a risk common to all TPO receptor agonists [10]. Therefore, oral and maxillofacial surgeons must communicate closely with gastroenterologists when considering performing tooth extraction surgery in conjunction with a regimen of lusutrombopag and should always consider the possibility of adverse events during the procedure.
Conclusion
Lusutrombopag was able to increase platelet count sufficiently to achieve haemostasis during tooth extraction in a patient with thrombocytopenia caused by chronic liver disease thus, negating the added physical burden that would have been imposed on the patient had the conventional method of platelet transfusion been required.
[1]. Schmidt KG, Rasmussen JW, Bekker C, Madsen PE, Kinetics and in vivo distribution of 111-In-labelled autologous platelets in chronic hepatic disease: mechanisms of thrombocytopeniaScand J Haematol 1985 34(1):39-46. [Google Scholar]
[2]. Afdhal N, McHutchison J, Brown R, Jacobson I, Manns M, Poordad F, Thrombocytopenia associated with chronic liver diseaseJ Hepatol 2008 48(6):1000-07. [Google Scholar]
[3]. Maan R, de Knegt RJ, Veldt BJ, Management of thrombocytopenia in chronic liver disease: focus on pharmacotherapeutic strategiesDrugs 2015 75(17):1981-92. [Google Scholar]
[4]. Poordad F, Review article: thrombocytopenia in chronic liver diseaseAliment Pharmacol Ther 2007 26(Suppl.1):5-11. [Google Scholar]
[5]. Neunert C, Lim W, Crowther M, Cohen A, Solberg L Jr, Crowther MA, The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopeniaBlood 2011 117:4190-207. [Google Scholar]
[6]. Kim ES, Lusutrombopag: First global approvalDrugs 2016 76(1):155-58. [Google Scholar]
[7]. Izumi N, Osaki Y, Yamamoto K, Kurokawa M, Tanaka K, Kano T, A phase 3, randomized, double-blind, placebo-controlled study of lusutrombopag for thrombocytopenia in patients with chronic liver disease undergoing elective invasive procedures in Japan (L-PLUS 1)Hepatology 2015 62(6 Suppl):19-20. [Google Scholar]
[8]. Pharmaceuticals and Medical Devices Agency [Internet]. Mulpleta® (lusutrombopag) tablets: Japanese prescribing information [article in Japanese]. Tokyo, Japan. [cited 3 Nov 2015]. Available from: https://www.pmda.go.jp/ [Google Scholar]
[9]. Shionogi [Internet]. Shionogi receives marketing and manufacturing approval in Japan for MULPLETA® tablets 3 mg for improvement of thrombocytopenia. Osaka, Japan. [cited 28 Sep 2015]. Available from: http://www.shionogi.co.jp/en/ [Google Scholar]
[10]. Nguyen TT, Palmaro A, Montastruc F, Lapeyre-Mestre M, Moulis G, Signal for thrombosis with eltrombopag and romiplostim: a disproportionality analysis of spontaneous reports within VigiBase®Drug Saf 2015 38(12):1179-78. [Google Scholar]