Introduction
Acute encephalitis is a clinical emergency which presents as a rapidly progressive encephalopathy (usually in less than 6 weeks) caused by brain inflammation [1]. The estimated incidence of encephalitis in high-income countries is about 5–10 per 100,000 inhabitants per year; people of all ages can be affected which represent a significant burden on patients, families and society [2,3]. The aetiology may be either infectious, parainfectious or non infectious such as autoimmune. Autoimmune conditions are not a common cause of encephalitis but with ongoing identification of novel autoantibodies and expansion of clinical spectra of disease, this entity is being increasingly recognised. Moreover it has shown a good response with steroids and immunomodulator therapy even in the absence of confirmed serology suggestive of autoimmune basis [4,5].
A variety of autoimmune causes have now been described including anti-LGI1 encephalitis (previously termed anti-Voltage-Gated potassium Channel ‘‘anti-VGKC’’ antibody encephalitis) and anti N-Methyl-D-aspartic Acid Receptor (anti-NMDAR) encephalitis [6,7].
The purpose of the present case series is to highlight the importance of early recognition of AE and its management. Hence, we present five cases of AE with variable clinical spectrum, its aetiology, management and outcome.
Case 1
A 16-year-old female presented in our neurology OPD with mild weakness in left side of body leading to buckling of knee for one day. After 3 days, she had 2 episodes of Generalised Tonic Clonic Seizures (GTCS). She was put on Valproate. Over the next 4 days she started developing oro-lingual-facial-distal upper limb dyskinesia and had difficulty in walking. These symptoms further increased with behavioural changes in form of anxiety, agitation, insomnia and short-term memory loss. There was no other relevant recent medical history. Past medical history revealed viral-like prodromes (fever, nausea, diarrhoea) occurring 3 weeks before psychiatric and/or neurologic symptoms leading to hospitalization. Family, medication, gynaecological and social history were unremarkable. Routine investigation including complete blood count, renal and liver function test, viral markers, urine microscopy was within normal limit. She was empirically given injectable acyclovir (10 mg/kg) in divided doses. MRI brain was normal. Electroencephalogram (EEG) showed diffuse slowing. Cerebrospinal Fluid (CSF) cytology showed lymphocytic pleocytosis with normal protein and serum–CSF glucose ratio. CSF-HSV PCR was negative. Presence of psychiatric symptoms with neurological signs like dyskinesia and seizures led to the search of autoantibodies. So, anti basal ganglia and anti-NMDA antibodies in CSF were suggested which came out to be strongly positive after 5 days. Acyclovir was stopped and injectable pulse Methylprednisolone (1g/day) was given for 5 days followed by oral prednisolone 1mg/kg. Computed Tomography (CT) pelvis was unremarkable. She started showing mild improvement in her behaviour and cognition but dyskinesia continued. She could walk with difficulty. Repeat EEG revealed diffuse slowing. Following 15 days when she showed no further improvement, second line immunotherapy with injectable pulse Cyclophosphamide was started after taking consent from her parents. After three weeks of pulse Cyclophosphamide therapy, there was marked improvement in cognition, gait, behaviour and abnormal movements. Repeat EEG and Magnetic resonance imaging (MRI) brain was normal. She completed six cycles of monthly pulse Cyclophosphamide. After six months she was completely normal and resumed her activities.
Case 2
An 18-year-old female was admitted to the psychiatry department, with confusion, delusion, insomnia, altered sleep pattern and short-term memory loss for one week. She was prescribed Haloperidol and Benzodiazepines. Initially there was mild improvement but five days later she exhibited bizarre behaviour, fluctuating consciousness level and was unable to recognise her parents. Past medical history was insignificant. She was shifted to ICU where she developed dysautonomic symptoms like fever and tachycardia. Over the next two days she developed oro-facial-distal limb dyskinesia. Then she was transferred to our neurology department. Her Glasgow Coma Scale (GCS) score continued to fall. Routine blood investigations were within normal limit. MRI brain and EEG were normal. CSF virology and its cytology were negative. Screening for toxins, auto antibodies and tumour markers did not yield any result. Her symptoms were potentially consistent with a primary psychiatric disorder, but the presence of neurologic features related to memory and cognition and dyskinesia prompted an alternative diagnosis. Samples were sent for anti-NMDA receptor antibodies, paraneoplastic (Anti-Hu, Anti-Yo, Anti-Ri, Anti-Ma 1/2, Anti-CRMP-5 and Anti-Ampiphysin) and VGKC antibodies out of which anti-NMDA receptor antibodies were strongly positive. Contrast-Enhanced Computed Tomography (CECT) thorax, abdomen and pelvis were done to screen for any paraneoplastic disorders. She was given five day course of daily pulse (1 gm/day) methylprednisolone (MPS) followed by oral prednisolone (1 mg/day) but no improvement was observed. A course of intravenous immunoglobulin (IV IG) (2 gm/day) over five days was given. There was some improvement in cognition, behaviour and dyskinesia. After one month she continued to have dyskinesia and fluctuation of consciousness. Second line of immunotherapy with pulse injectable Cyclophosphamide was started. Following one month all her symptoms subsided. She continued to have six cycles of monthly pulse Cyclophosphamide. At one year of regular follow up she is asymptomatic.
Case 3
A 30-year-old male had 3 to 4 episodes of GTCS within five days. He was admitted to neurology department with status epilepticus with postictal psychosis with no significant past or medical history. He was given injectable Lorazepam followed by phenytoin and valproate. EEG revealed diffuse slowing. MRI brain was normal. He continued to have recurrence of seizure the following day for which Levetiracetam was added. Though frequency and duration of seizures decreased, he did not recover from postictal psychosis. He was treated with Haloperidol, Promethazine but his psychosis did not improve. He used to thrash things, say abusive language to people. He was then put on midazolam infusion for 24 hours during which he had no seizure episodes. Two days later he started having oro facial limb dyskinesia with non-fluent aphasia. He was investigated in the context of encephalitis probably of viral or autoimmune in aetiology. CSF examination was normal and was negative for viruses. He was positive for CSF anti NMDA receptor antibodies. CECT thorax, abdomen and pelvis were done to screen for underlying malignancy. Pulse MPS (1 gm/day) and IV IG (2 gm/kg) was given over five days followed by oral prednisolone 60 mg/day. He showed improvement in dyskinesia, psychosis and speech but fluctuation of consciousness persisted. This continued for 15 days after which he was given pulse Cyclophosphamide. One month later he was fine. Monthly pulse Cyclophosphamide was continued for six months. He was normal as before and was on regular follow up. Six months later he presented to us with relapse in form of short term memory loss and non fluent aphasia. He was given pulse MPS for five days followed by injectable rituximab 1000 mg/day. He responded well and is on regular follow up.
Case 4
An 80-year-old diabetic, hypertensive male presented with subacute onset of short term memory loss, disorientation, cognitive decline and trans cortical aphasia for one month. He was admitted in medicine department. History revealed chronic kidney disease. On third day of hospitalization he became more aggressive, restless and developed mutism. He only produced incomprehensible sounds. He was investigated on the line of viral encephalitis. Routine investigation was normal except raised creatinine level (6 mg/dl) and microcytic hypochromic anaemia. MRI brain was normal. CSF showed lymphocytic pleocytosis (26 white cells/ml of which 70% were lymphocytes), protein 0.60 gm/L and normal blood – CSF sugar ratio. CSF virology was negative. Injectable acyclovir (10mg/day) in divided doses was empirically started. On day 15 of hospital in care, he developed right sided faciobrachial dystonic seizures. Then he was transferred to neurology department where he was suspected to be a case of AE based on the clinical history and course of disease. He was started with pulse MPS for five days. IV IG could not be given as renal function was comprised. Meanwhile sample for detection of anti-NMDA receptor antibodies, paraneoplastic (Anti-Hu, Anti-Yo, Anti-Ri, Anti-Ma 1/2, Anti-CRMP-5 and Anti-Ampiphysin) and VGKC antibodies was sent which later came out to be positive for LGI 1 antibodies. Second line immunotherapy with rituximab was planned as pulse Cyclophosphamide could not be given due to deranged renal function. His condition deteriorated further. Over next two days urine output decreased and he became unconscious. Arterial Blood Gas (ABG) showed metabolic acidosis, hyponatremia (114 mmol/l). He was then put on ventilator but he succumbed to his illness.
Case 5
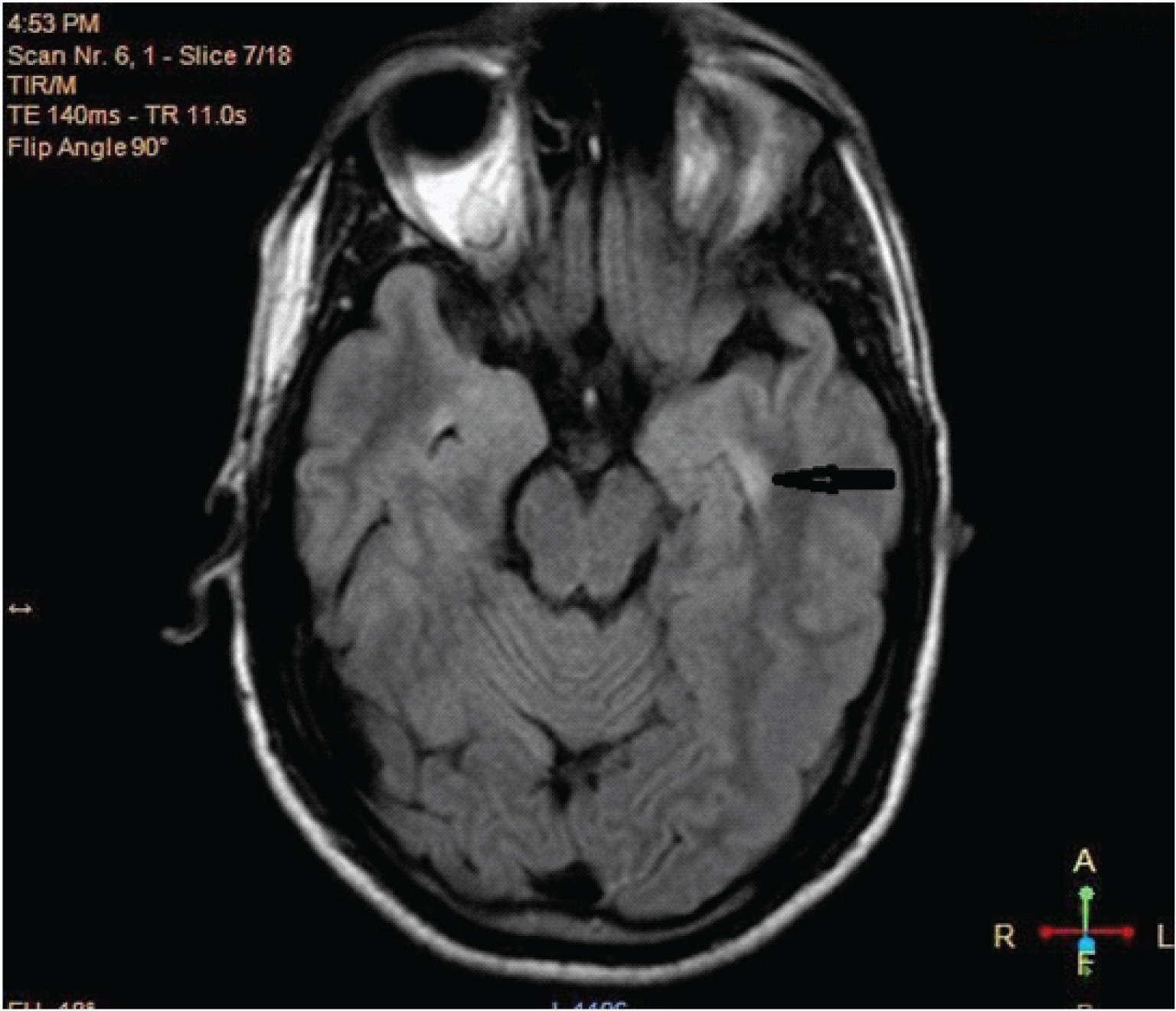
A 65-year-old female presented to us with cognitive decline and oro facial dyskinesia since 25 days with no significant past or medical history. Five days after admission she developed GTCS. Routine blood chemistry was normal and viral markers were negative. EEG showed background of slowing in both hemispheres. MRI brain T2 flair image [Table/Fig-1] showed hyperintense signal in left medial temporal lobe. CSF showed lymphocytic pleocytosis (19 white cells/mL of which 76% were lymphocytes) and was negative for virology. She was suspected to be a case of encephalitis probably autoimmune in origin based on coexistence of psychiatric and neurolologic symptoms. Meanwhile sample for anti-NMDA receptor antibodies, paraneoplastic (Anti-Hu, Anti-Yo, Anti-Ri, Anti-Ma 1/2, Anti-CRMP-5 and Anti-Ampiphysin) and VGKC antibodies was sent which later came out to be negative. CT throrax abdomen and pelvis was negative for any malignancy. She was given pulse MPS for five days and was put on monthly injectable Cyclophosphamide. One month later her cognition, dyskinesia and seizures improved. She was continued on six cycles of Cyclophosmide. After one year of follow up she was doing well.
MRI brain T2 weighted Flair image showing hyperintense signal in left medial temporal lobe indicated by black arrow.
Discussion
Encephalitis continues to result in significant morbidity and mortality worldwide. In a record based study of the entire population of India from 1978 to 2011, there were 125,030 cases of Acute Encephalitis Syndrome (AES) [8] in which the cause was unknown in 68-75% [9,10]. In the western population of California, USA, a study on 1500 cases of encephalitis revealed a confirmed aetiological agent (the majority of these were viral followed by bacterial) in only 16%; a suspected aetiological agent in 13%; and a noninfectious cause (autoimmune disease and vasculitis being the most common) in 8% cases. No aetiology was found in nearly 75 %cases referred to specialist units [11].
This emphasizes the need to keep AE as a differential diagnosis when infectious aetiology has been ruled out. A careful history, examination and relevant investigations can give early clues regarding the aetiology. Serology for neuronal autoantibodies can further aid to the correct diagnosis. In the above cases, encountered within a 20-month period, we illustrate the spectrum of presentations of varying types of AE. We report 5 patients with AE, representing the first case series of AE in people of western Rajasthan. Cases 1, 2 and 3 describe two adolescent females and a young male respectively with anti-NMDAR encephalitis. Case 4 describes an elderly male with anti-LGI1 encephalitis. Case 5 describes an elderly female with non-infectious, presumed AE that responded well to immunomodulatory therapies although no autoimmune antibody was identified.
A variety of autoimmune causes of encephalitis with specific signs and symptoms have been described leading to the early diagnosis of AE [Table/Fig-2]. Though many case reports of AE have been published and research articles have been reported from western countries, only 2 case series [12,13] have been reported from India to our knowledge in which anti NMDA AE was found to be most common. Pandit AK et al., found that seizure is the predominant clinical presentation in 100% of patients who responded well to immunotherapy (67% were seizure free at follow up) concluding that rare causes of status epilepticus, epilepsy, and cognitive decline may be immune related and a high index of suspicion should be kept, as these are reversible in many cases [13].
Clinical findings of associated autoantibody disorders.
| Clinical fnding | Associated autoantibody disorders |
|---|
| Psychosis | NMDAR, AMPAR, GABA-B-R |
| Dystonia, chorea | NMDAR, Sydenham chorea, D2R |
| Hyperekplexia | GlyR |
| Status epilepticus | Most characteristic of GABA-B-R and GABA-A-R but NMDAR is much more common;may occur in other types as well |
| New onset type 1 diabetes | GAD65 |
| Fasciobrachial dystonic seizures | LGI1 |
| Neuromyotonia, muscle spasms, fasciculations | Caspr2 |
| Stiff-person syndrome and/or exaggerated startle | GAD65, GlyR, Amphiphysin (with GAD65 being most common in stiff person/stiff limb and GlyR in PERM, and Amphiphysin in women with breast cancer) |
| CNS (myoclonus, startle, delirium) and gastrointestinal hyper-excitability | DPPX |
| Cranial neuropathies | Ma2, Hu, Miller-Fisher, Bickerstaff (but also infections like Sarcoidosis, Lyme, TB) |
| Cerebellitis | GAD65, PCA-1 (Yo), ANNA-1 (Hu), DNER (Tr), mGluR1, VGCC |
In our series, anti NMDAR encephalitis was most common. Seizures and status epilepticus were the presenting clinical feature in case 1 and 3 respectively, memory and cognitive impairment associated with personality change was predominant in case 2, case 4 initially presented with viral encephalitis like picture but later developed faciobrachial dystonic seizures typical of LG1 encephalitis, case 5 presented with cognitive impairment, confusion and abnormal movements.
Psychiatric presentation is early in the course of AE. Maat P et al., in his study concluded that 80% anti-NMDAR encephalitis patients presented with psychiatric symptoms initially [14]. Anti-AMPAR and anti-GABA-B-R [15] can have early prominent psychiatric manifestations.
Secondly, abnormal movements like dystonia or chorea can be the presenting symptom in several types of AE like anti-NMDAR encephalitis [16]. Thirdly, seizures may be the presenting symptom in AE. Seizures may occur at any stage of anti-NMDA receptor (anti-NMDAR) encephalitis. Anti GABA-B and GABA-A receptors encephalitis carry a high risk of severe seizures and intractable status epilepticus [17,18].
Most forms of AE respond to immune therapies. Anti-NMDAR encephalitis patients usually respond within weeks to first-line treatments- corticosteroids and IV IG or plasma exchange (PE). A total of 5-6 PE are given every alternate day with albumin and normal saline as a replacement fluid [19].
Early treatment results in good outcome in 80% of patients [20]. For the 47% of patients who do not respond to first-line treatments [20], a second-line immunotherapy: rituximab or Cyclophosphamide or both are advised. The outcome of the second-line immunotherapy in patients is improved in 65% of cases [21,22]. Despite this second-line treatment, relapse can occur in 20%–25% of the case [7,23,24].
In our series, all cases received combined first and second line immunotherapy except case 4. Case 3 relapsed with decreased speech output and facio-limb dyskinesias and was treated with second line agent like Rituximab.
We achieved complete seizure freedom in all cases and overall there was significant improvement in the neurological status in all our patients. Our series reflects that the diagnosis of autoimmune aetiology should be considered in all persons with an unknown aetiology of either status epilepticus or sub-acute onset of cognitive decline and behaviour changes when other common causes have been ruled out and we should be highly suspicious when neurological and psychiatric symptoms are coexisting.
CSF lymphocytic pleocytosis was observed in all our patients similar to Dalmau’s series in which 95 CSF studies were abnormal, in form of raised protein and lymphocytic pleocytosis. We had normal MRIs in anti NMDAR antibody positive cases whereas Dalmau’s series had about 55% abnormal MRIs in the form of basal ganglia atrophy, periventricular hyperintensities, and bilateral limbic region hyperintensity on T2 and FLAIR images [22]. In our cases we did not find any evidence of malignancy either at the time of presentation or at 1 year of follow up.
We had one elderly male patient of autoimmune epilepsy with antiVGKC antibody positivity. MRI brain was normal and CSF showed lymphocytic pleocytosis with mildly raised protein. In a series of 51 cases by Tan KM et al., predominant clinical features observed were cognitive impairment in 71%, seizures (faciobrachial) in 58%, autonomic instability in 33%, myoclonus in 29%, sleep disturbances in 26%, and extrapyramidal features in 21% (chorea in 4% cases) cases [25]. Additionally hyponatremia, cranial nerve or brainstem involvement, peripheral nerve hyperexcitability, neuropathy, headache, cerebellar features, and Morvan syndrome were also noted in decreasing order. On investigations, 57% CSF (27 of 47 available CSF studies) and 54% (26 of 48 available MRIs) of MRIs were abnormal.
We had one elderly female patient without any positive serology presenting with seizures, cognitive impairment, subacute onset of memory loss. CSF showed lymphocytic pleocytosis, EEG showed diffuse slowing and MRI brain revealed left medial temporal lobe hyperintensity on T2 and FLAIR images. She responded favourably to immunotherapy—a response which was maintained—indicating a likely autoimmune cause for the encephalitis. There are further cases in the literature reporting patients presenting with features of AE, negative anti-LGI1 and anti-NMDA antibodies but full response to immunotherapy with IV IG and steroids [26].
The most possible explanation could be that there are other conditions which can cause AE in addition to anti-NMDAR, anti-LGI1or CASPR2; which are Anti-AMPA, Anti-GABA and the intracellular antigens anti-Hu and anti-Ma2 (one of the causes of limbic encephalitis)- our patient could have one of these.
However, in patients with normal MRI brain, 18F Fluorodeoxyglucose (FDG), Positron Emissiontomography (PET) [27], 99mTc-d, L-hexamethyl-propyleneamine oxime (HMPAO) Single Photon Emission Computed Tomography (SPECT) [28] can aid to early diagnosis by showing variable multifocal abnormalities in cortical and subcortical areas changing during the course of the disease.
Extensive study requiring brain biopsies of the temporal and limbic areas can hardly confirm any aetiology as they show non-specific changes like neuronal loss, astrogliosis, and mononuclear cell infiltrates.
Lastly, one should need to rule out other autoimmune causes of encephalitis which are paraneoplastic, ADEM, multiple sclerosis (MS), lupus, Bickerstaff encephalitis and Miller Fisher syndrome (MFS). Serologies for lupus and paraneoplastic antibodies including anti-Hu, anti Ma1, anti Ta, anti Tr, anti Ri, anti Yo, amphyphisin, collapsing response-mediator protein 5 are helpful [29].
Detection of GQ1b antibodies with clinical features like areflexia in MFS and ataxia, ophthalmoplegia in Bikerstaff encephalitis are helpful.
However, besides other autoimmune causes of encephalitis, HSV encephalitis can itself trigger anti NMDAR encephalitis probably due to molecular mimicry or altering the levels of NMDAR by virus induced damaged neurons which may benefit from immunotherapy [30].
Conclusion
Although AE can present in a similar way to other encephalitic processes—such as those of an infectious aetiology, they do express some distinct characteristic features. One must keep the AE as a differential diagnosis, in whom aetiology has not been confirmed. The excellent response to treatment in four out of our five patients also emphasizes an urge for prompt consideration, investigation and recognition of AE. Early treatment, initially with steroids in addition to IV IG, plasma exchange and in refractory cases, Cyclophosphamide and Rituximab is necessary for rapid clinical outcome. Thus, our purpose is to increase the awareness of the clinical spectrum of AE in order to attain good clinical recovery through early diagnosis and management of this treatable disorder.
[1]. Venkatesan A, Tunkel AR, Bloch KC, Lauring AS, Sejvar J, Bitnun A, International Encephalitis Consortium. Case definitions, diagnostic algorithms, and priorities in encephalitis: consensus statement of the international encephalitis consortiumClin Infect Dis 2013 57(8):1114-28. [Google Scholar]
[2]. Jmor F, Emsley HC, Fischer M, Solomon T, Lewthwaite P, The incidence of acute encephalitis syndrome in Western industrialised and tropical countriesVirol J 2008 5:134-46. [Google Scholar]
[3]. Vora NM, Holman RC, Mehal JM, Steiner CA, Blanton J, Sejvar J, Burden of encephalitis-associated hospitalizations in the United States, 1998-2010Neurology 2014 82(5):443-51. [Google Scholar]
[4]. Urbach H, Soeder BM, Jeub M, Klockgether T, Meyer B, Bien CG, Serial MRI of limbic encephalitisDiag Neurorad 2006 48(6):380-86. [Google Scholar]
[5]. Bataller L, Kleopa KL, Wu GF, Rossi JE, Rosenfeld MR, Dalmau J, Autoimmune limbic encephalitis in 39 patients: immunophenotypes and outcomesJ Neurol Neurosurg Psychiatry 2007 78(4):381-85. [Google Scholar]
[6]. Anderson NE, Barber PA, Limbic Encephalitis – A reviewJ Clin Neurosci 2008 15(9):961-71. [Google Scholar]
[7]. Florance NR, Davis RL, Lam C, Szperka C, Zhou L, Ahmad S, Anti-NMDA-receptor encephalitis in children and adolescentsAnn Neurol 2009 66(1):11-18. [Google Scholar]
[8]. Potharaju NP, Incidence rate of acute encephalitis syndrome without specific treatment in India and NepalIndian J Community Med 2012 37(4):240-51. [Google Scholar]
[9]. AES/JE Cases and Deaths in the Country. National Vector Borne Disease ControleProgramme. Directorate General of Health Services. Ministry of Health and Family Welfare, Government of India. 2012 [Google Scholar]
[10]. Director, Child Health Division. Teku, Kathmandu, Nepal: Department of Health Services, Ministry of Health and Population; 2012. Acute Encephalitis Syndrome/Japanese Encephalitis data of Nepal [Google Scholar]
[11]. Glaser CA, Honarmand S, Anderson LJ, Schnurr DP, Forghani B, Cossen CK, Beyond viruses: clinical profiles and etiologies associated with encephalitisClin Infect Dis 2006 43:1565-77. [Google Scholar]
[12]. Jacob S, Autoimmune encephalitis – The evolving spectrum: An Indian case seriesNeurology India 2015 63(5):647-49. [Google Scholar]
[13]. Pandit AK, Ihtisham K, Garg A, Autoimmune encephalitis: A potentially reversible cause of status epilepticus, epilepsy, and cognitive declineAnn Indian Acad Neurol 2013 16(4):577-84. [Google Scholar]
[14]. Maat P, de Graaff E, van Beveren NM, Hulsenboom E, Verdijk RM, Koorengevel K, Psychiatric phenomena as initial manifestation of encephalitis by anti-NMDAR antibodiesActa Neuropsychiatr 2013 25(3):128-36. [Google Scholar]
[15]. Höfberger R, Titulaer MJ, Sabater L, Dome B, Rózsás A, Hegedus B, Encephalitis and GABAB receptor antibodies: novel fndings in a new case series of 20 patientsNeurology 2013 81(17):1500-06. [Google Scholar]
[16]. Armangue T, Titulaer MJ, Málaga I, Bataller L, Gabilondo I, Graus F, Pediatric anti-Nmethyl-D-aspartate receptor encephalitis-clinical analysis and novel findings in a series of 20 patientsJ Pediatr 2013 162(4):850-56. [Google Scholar]
[17]. Lancaster E, Lai M, Peng X, Hughes E, Constantinescu R, Raizer J, Antibodies to the GABA(B) receptor in limbic encephalitis with seizures: case series and characterisation of the antigenLancet Neurol 2010 9(1):67-76. [Google Scholar]
[18]. Petit-Pedrol M, Armangue T, Peng X, Bataller L, Cellucci T, Davis R, Encephalitis with refractory seizures, status epilepticus, and antibodies to the GABAA receptor: a case series, characterisation of the antigen, and analysis of the effects of antibodiesLancet Neurol 2014 13(3):276-86. [Google Scholar]
[19]. Pham HP, Daniel-Johnson JA, Stotler BA, Stephens H, Schwartz J, Therapeutic plasma exchange for the treatment of anti-NMDA receptor encephalitisJournal of Clinical Apheresis 2011 26(6):320-25. [Google Scholar]
[20]. Viaccoz A, Desestret V, Ducray F, Picard G, Cavillon G, Rogemond V, Clinical specifcities of adult male patients with NMDA receptor antibodies encephalitisNeurology 2014 82(7):556-63. [Google Scholar]
[21]. Titulaer MJ, Mccracken L, Gabilondo I, Armangué T, Glaser C, Iizuka T, Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort studyLancet Neurol 2013 12(2):157-65. [Google Scholar]
[22]. Dalmau J, Lancaster E, Martinez-Hernandez E, Rosenfeld MR, Balice-Gordon R, Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitisLancet Neurol 2011 10(1):63-74. [Google Scholar]
[23]. Irani SR, Bera K, Waters P, Zuliani L, Maxwell S, Zandi MS, N-methyl-D-aspartate antibody encephalitis: temporal progression of clinical and paraclinical observations in a predominantly non paraneoplasticBrain 2010 133(Pt 6):1655-67. [Google Scholar]
[24]. Gabilondo I, Saiz A, Galán L, González V, Jadraque R, Sabater L, Analysis of relapses in anti-NMDAR encephalitisNeurology 2011 77(10):996-99. [Google Scholar]
[25]. Tan KM, Lennon VA, Klein CJ, Boeve BF, Pittock SJ, Clinical spectrum of voltagegated potassium channel autoimmunityNeurology 2008 70(20):1883-90. [Google Scholar]
[26]. Modoni A, Masciullo M, Spinelli P, Marra C, Tartaglione T, Andreetta F, Successful treatment of acute autoimmune limbic encephalitis with negative VGKC and NMDAR antibodiesCog Behav Neurol 2009 22(1):63-66. [Google Scholar]
[27]. Maeder-Ingvar M, Prior JO, Irani SR, Rey V, Vincent A, Rossetti AO, FDG-PET hyperactivity in basal ganglia correlating with clinical course in anti-NDMA-R antibodies encephalitisJ Neurol Neurosurg Psychiatry 2011 82(2):235-36. [Google Scholar]
[28]. Llorens V, Gabilondo I, Gómez-Esteban JC, Agundez M, Mendibe M, Bergara JC, Abnormal multifocal cerebral blood flow on Tc-99m HMPAO SPECT in a patient with anti-NMDA-receptor encephalitisJ Neurol 2010 257(9):1568-69. [Google Scholar]
[29]. Xia Z, Mehta BP, Ropper AH, Kesari S, Paraneoplastic limbic encephalitis presenting as a neurological emergency: A case reportJ Med Case Reports 2010 4:95 [Google Scholar]
[30]. Prüss H, Finke C, Höltje M, Hofmann J, Klingbeil C, Probst C, N-Methyl-D-Aspartate receptor antibodies in herpes simplex encephalitisAnn Neurol 2012 72(6):902-11. [Google Scholar]