ZIKV is a mosquito-borne, positive-strand RNA flavivirus. According to both Foy BD et al., and Lazeric S, it was first isolated in Southeast Asia and Africa in 1947, from sentinel monkeys and sick persons, thus having an African and Asian lineage [1,2]. In 2007, the Asian lineage of ZIKV emerged as a ZIKV outbreak in Yap Island infecting approximately 70% of their population [1]. This outbreak made Yap Island the first place outside Asia and Africa to detect ZIKV [3]. To date, since its reoccurrence, ZIKV has continuously spread and cases have been reported in 67 countries and territories [4].
ZIKV commonly presents with fever, maculopapular rash, conjunctivitis, and arthralgia while headache, vomiting and oedema are clinically seen in less cases [5]. Most cases (60%-80%) remain asymptomatic [2]. Furthermore, ZIKV has been associated with adverse pregnancy outcomes such as microcephaly, brain and placental calcifications [6], Central Nervous System (CNS) abnormalities in newborns of infected mothers and foetal death [7], suggesting it to be a teratogenic [8,9].
In infected persons, ZIKV RNA may be present in a variety of bodily fluids such as saliva, urine, blood, cerebrospinal fluid and amniotic fluid [14]. Presence of ZIKV RNA in the body confirms the diagnosis [2]. Evidence shows ZIKV RNA to be present in semen and urine for longer periods, upto 10 days from symptom onset, in comparison to blood and saliva, first three to five days from the onset of symptoms [2,15]. Research is limited on the replication of ZIKV in the genital tract, and the persistence of the virus in the body is currently unclear [16].
Testing for ZIKV depends on the time period since the onset of symptoms. Within the first seven days from onset of symptoms, it is recommended to use Nucleic Acid Testing (NAT) on serum and urine [2]. For patients who present onset of symptoms over seven days, serology for ZIKV-specific Immunoglobin M (IgM) antibody detection is recommended since, viraemia significantly decreases after a week from onset of symptoms meanwhile antibodies are being developed by body [2,9,15].
The aim of this study was to conduct a literature review about ZIKV infection and its transmission methods including vector-borne, sexual contact, bodily fluids, blood transfusion intrauterine vertical transmission (non-vector-borne) and breast milk transmission, using recent research studies to better understand how ZIKV may spread.
Materials and Methods
A systematic review of the literature on cases with mode of transmission of ZIKV was performed using the electronic databases EBSCO, EMBASE, Google Scholar, PubMed, Web of Science and MEDLINE. Reviewed articles were published after the most recent ZIKV outbreak in 2007 until the present time, December 2016.
During the search process, key phrases used included: Zika virus, transmission, mosquito-borne transmission, sexual transmission, person-to-person transmission, countries at risk of Zika virus transmission, microcephaly and Zika virus, and transmission through bodily fluids. Approximately, 1907 articles were published between 2007 and 2016 using the above key phrases in database searches and other sources.
Inclusion criteria were scholarly case report studies, case series studies, cohort studies, review studies, and case control studies detailing the mode of transmission of ZIKV, articles in which ZIKV cases were confirmed and of human subjects, and in the English language. Articles were not limited to a specific geographical location or adult and gestational age.
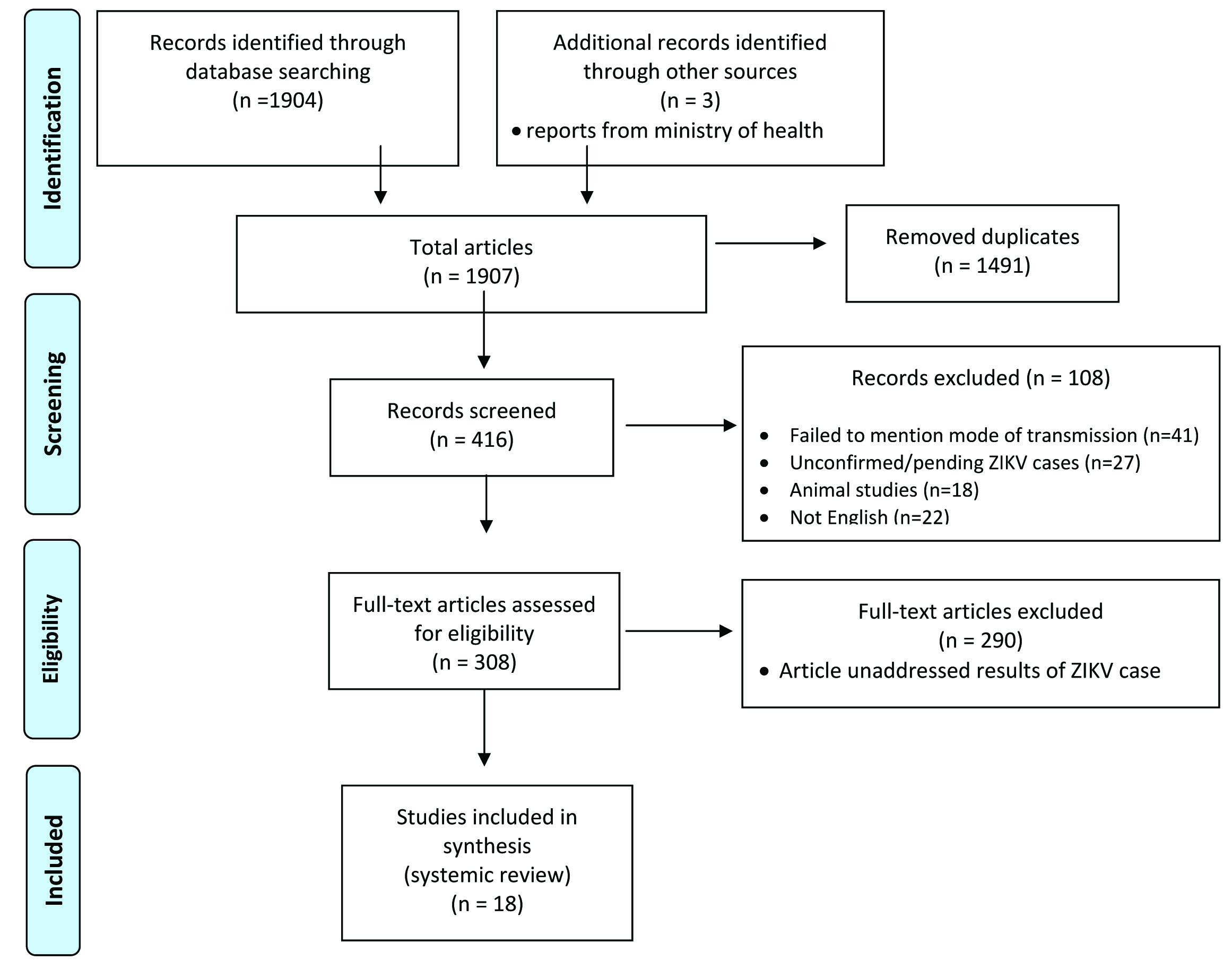
Based on their relevance to the criteria of interest, only 18 articles were carefully reviewed in order to better understand different methods of transmission of ZIKV. All reviewed articles involved only human subjects who demonstrated ZIKV clinical symptoms, positive tested samples (urine, serum, saliva and/or semen) and/or antibody for ZIKV. About 18 articles that were included in the final selection, nine articles provided information on more than one possible mode of transmission, explaining why one and the same article is included under several subcategories. [Table/Fig-1] summarizes the process in which the articles were selected for this review.
Flow chart presented the screening of articles on cell phone and its effect on orofacial structures, in medline and pubmed database to be included in the review.
Results
A total of 18 articles were selected and reviewed. About nine articles were related to the transmission of ZIKV through sexual contact in which eight out of the nine include travel-associated cases [16-24], one article exclusively discussesed local mosquito-borne transmission of ZIKV [25], seven articles were directly related to the vertical transmission of ZIKV from mother to foetus with one including a breastmilk transmission case [6,7,13,26-29] and one article exclusively discussing breast milk transmission to the newborn [29]. Several articles include multiple cases involving different methods of transmission. For the purpose of this review, the cases have been separated and allocated to the appropriate method of transmission. This systemic review included a total of 2573 ZIKV infected persons. Vector-borne transmission was the highest mode of transmission with 95.34% of the total infected followed by intrauterine vertical transmission with 3.15% of infected foetuses.
Sexual Transmission
Sexual transmission was the third most common method of transmission in our systemic review. A total of nine articles accumulating 36 cases reporting ZIKV infection through sexual transmission were reviewed. As indicated in [Table/Fig-2], all individuals demonstrated clinical symptoms, which was the reason for seeking medical attention. All infected persons had previous sexual contact with someone who recently traveled to a country with ZIKV transmission. Most common sexual transmission is seen between male and his partner (female or male), however one article by Davidson A et al., showed the first suspected female to male transmission in 2016 [17]. All cases reported condomless vaginal sexual intercourse between the male and female partners; however one case reported male to male condomless sexual contact via anal sexual intercourse [18].
Summary of different methods of transmission of ZIKV and their findings from studies reviewed in systematic review*.
| Method of transmission of ZIKV | Study | Study type | Time and Location | Number of infected subjects | Symptoms | Key Findings |
|---|
| Sexual transmissionInvolving sexual contact with already ZIKV infected person | Deckard D et al., [18] | Case series | January 2016; Texas, USA | 1 male | Headache, lethargy, malaise, fever, myalgia, rash, conjunctivitis | Tested positive for IgM responses for ZIKV in serum. Undetected in urine or saliva Partner returned from Venezuela Condomless insertive anal sex with male partner
|
| Hills SL et al., [16] | Case series | January 2016; Continental US | 2 females | Case 1: Fever, rash, conjunctivitis, myalgiaCase 2: fever, rash, arthralgia, eye pain, photophobia, headache, vomiting, myalgia | Case 1: ZIKV detected in serum.Partner traveled to CaribbeanCase 2: ZIKV detected in serum.Partner returned from Central AmericaBoth individuals engaged in condomless vaginal sex |
| Brooks R et al., [19] | Case series | June 2016; Maryland, USA | 1 female | Fever, rash | ZIKV present in urine, absent in serum Partner returned from Dominican Republic Condomless vaginal sex
|
| Fréour T et al., [20] | Case series | April 2016; France | 1 female | Asymptomatic. Tested for the purpose of In Vitro Fertility procedure | ZIKV detected in serum and urine Partner returned from Martinique
|
| Davidson A et al., [17] | Case series | 2016; NYC, USA | 1 male | Rash, fever, arthralgia, conjunctivitis | ZIKV detected in urine, absent in serum Partner returned from area of ZIKV transmission Condomless vaginal sex
|
| Armstrong P et al., [23] | Case series | January 1-February 16, 2016; USA | 5 | Rash, fever, arthralgia | Confirmed ZIKV |
| Musso D et al., [22] | Case report | December 2013; Tahiti | 1 male | Asthenia, fever, arthralgia, hematospermia | ZIKV detected in semen and urine, absent in blood suspected sexual transmission
|
| Frank C et al., [21] | Case series | April 2016 | 1 female | URTI, swollen neck lymph nodes, rash | ZIKV detected in urine and serum. No IgM antibody present Partner returned from Puerto Rico
|
| Walker W et al., [24] | Case series | January 1-July 31; 50 states and DC | 23 | Not specified | Confirmed ZIKV |
| Travel-Associated (Vector-borne)Involves living in or traveling to area of ZIKV transmission containing mosquitoes | Deckard D et al., [18] | Case series | January 2016; Texas, USA | 1 male | Fever, rash, conjunctivitis | Tested positive for IgM responses for ZIKV Ambiguous results found in semen Traveled to Venezuela
|
| Hills SL et al., [16] | Case series | January 2016; Continental US | 3 males | Case 1: Fever, rash, conjunctivitis, arthralgiaCase 2: fever, arthralgia, eye discomfort, pruritus, myalgiaCase 3: fever, rash, arthralgia, conjunctivitis, myalgia, headache | Case 1: ZIKV results pending at time of publication. Traveled to the CaribbeanCase 2: Tested positive for IgM antibody, ZIKV confirmation pending at time of publicationTraveled to Central AmericaCase 3: ZIKV results pending at time of publicationTraveled to Central America |
| Brooks R et al., [19] | Case series | June 2016; Maryland, USA | 1 male | Asymptomatic | Serum tested positive for IgM antibody, absent in serum Traveled to Dominican Republic
|
| Fréour T et al., [20] | Case series | April 2016; France | 1 male | Asymptomatic. Tested for the purpose of In Vitro Fertility procedure | ZIKV detected in urine and seminal plasma, absent in serum Traveled to Martinique
|
| Davidson A et al., [17] | Case series | 2016; NYC, USA | 1 female | Headache, abdominal cramping, fever, fatigue, rash, myalgia, arthralgia, back pain, swelling in extremities, numbness and tingling in hands and feet, heavier menses | ZIKV detected in urine and serum |
| Armstrong P et al., [23] | Case series | January 1-February 16, 2016; USA | 110 | Rash, fever, arthralgia | Confirmed ZIKV All traveled to areas with active ZIKV transmission
|
| Frank C et al., [21] | Case series | April 2016 | 1 male | Fatigue, arthralgia, rash, URTI, swollen neck lymph nodes | Serum positive for IgM antibody Traveled to Puerto Rico
|
| Walker W et al., [24] | Case series | January 1-July 31, 2016; 50 states and DC | 2331 | Not specified | Confirmed ZIKV All reported travel to ZIKV affected area
|
| Likos A et al., [25] | Case series | July 2016; Florida, USA | 4 | All exhibited fever, rash, arthralgia for all 4 cases | Case A: ZIKV detected in serum and urineCase B: ZIKV detected in serum and urineCase C: results pending at the time of publicationMosquito larval development sites found close to workplaceCase D: Testing not mentionedMosquito larval development sites found close to workplace |
| Blood transfusionInvolves receiving donor blood | Barjas-Castro M et al., [30] | Case report | March 2015; Brazil | 1 | Asymptomatic | ZIKV detected in serum four days after blood transfusion. Suspected transmission through donated blood |
| Mother to foetus vertical transmissionZIKV intrauterine transmission from the mother to the foetus | Oliveira Melo A et al., [26] | Case series | 2016; Paraíba, Brazil | 2 pregnant women | Women suffered unspecified ZIKV symptoms and were diagnosed with fetal microcephaly | Both cases tested negative for ZIKV in serum, but tested positive in amniocentesis studies.Case 1: foetal femur length, abdominal circumference and transcranial Doppler was normal. Foetal brain demonstrated several brain anomalies including: brain atrophy, calcification in the white matter of the frontal lobes.Case 2: foetal femur length was normal, abdominal circumference was below normal. Foetal brain demonstrated several brain anomalies including failure to develop thalami, brain calcifications |
| Besnard M et al., [13] | Case series | 1: December 20132: February 2014;French Polynesia | 2 pregnant women | Case 1: mild pruritic rash starting 2 days before delivery, lasting 2 days after deliveryCase 2: gestational diabetes and intrauterine growth restriction | Both women were ZIKV positive in serum analysis. Newborns had a similar serum ZIKV RNA load.Case 1: Mother recovered well and foetus was stable. Newborn was asymptomatic. Case 2: Newborn had maculopapular rash, thrombocytopenia and low birth weight. The mother had a mild fever and puritic rash and myalgia |
| Mlakar J et al., [6] | Case report | October 2015; Ljubljana, Slovenia | 1 woman | At 13 weeks gestation, the case had a fever, retroocular and musculoskeletal pain, itching and a rash | At 32 weeks gestation, the foetus experienced microcephaly, growth retardation, calcifications in the placenta and the brain and mild ventriculomegalyFoetal autopsy (after pregnancy termination): microcephaly, open Sylvian fissures and small cerebellum and brain stem and the complete ZIKV sequence found in foetal brain tissue |
| Brasil P et al., [7] | Cohort study | September 2015 to February 2016; Rio de Janeiro, Brazil | 72 infected pregnant women | Only women with maculopapular rashes participated in the study | 72 of 88 women tested were positive for ZIKV (60 in serum, 46 in urine and 34 in both). 42 had further prenatal ultrasonographic exams but 12 were abnormal (and 2 foetal deaths). Foetuses showed signs of intrauterine growth restriction, cerebral calcifications, CNS modifications, oligohydramnios and anyhydramnios, abnormal arterial flow in cerebral or umbilical arteries
|
| Calvet G et al., [27] | Case study | September, 2015; Paraíba, Brazil | 2 pregnant women | Both women had fever, myalgia, rash and had been diagnosed with foetal microcephaly through amniocentesis | ZIKV genome was detected in the amniotic fluid of both women ZIKV was not found in the urine or serum Both cases demonstrated foetal microcephaly and several cerebellar malformations including cerebellar hypolplasia, ventriculomegaly and hemisphere asymmetry
|
| Driggers R et al., [28] | Case report | November 2015;travelled to Mexico, Guatemala and Belize | 1 pregnant woman | Woman experienced ocular pain, myalgia, mild fever and rash | Postmortem fetal studies of the foetal brain show apoptosis of neurons in the neocortex High ZIKV levels in the foetal brain, placenta and the umbilical cord. Low ZIKV levels were found in the foetal liver, muscle, lungs, spleen and amniotic fluid
|
| Perez S et al., [29] | Case report | 2016, Spain | 1 pregnant woman | Woman experienced a skin rash during pregnancy | ZIKV IgG, IgM and RNA were found in the maternal serum ZIKV was found in the amniotic fluid, umbilical cord and foetal brain tissue Foetal autopsy demonstrated foetal malformations and hydrocephalus, brain calcifications and arthrogryposis multiplex congenital Microcephaly was not detected and the thoracic and abdominal circumferences were normal
|
| Breast feedingZika transmission through infected breast milk | Dupont-Rouzeyrol M et al., [31] | Case report | July 2015; New Caledonia | 1 woman | After birth, the woman experienced a maculopapular rash | The mother’s serum and breastmilk were positive for ZIKVInfant serum was ambiguous |
| Besnard M et al., [13] | Case series | 1: December 20132: February 2014;French Polynesia | 2 pregnant women | Case 1: mother had mild pruritic rash starting two days before delivery, lasting two days after deliveryCase 2: mother had gestational diabetes and intrauterine growth restriction | Both women were ZIKV positive in serum analysis. Newborns had a similar serum ZIKV RNA load.Both cases showed positive results for presence of ZIKV RNA in breast milk but no replicative ZIKV was detected in culture |
*Note: several articles included multiple methods of transmission and infected persons have been separated in this table to their respective method of transmission.
In the articles specifying which bodily fluids were tested for the ZIKV, 3 individuals tested positive for ZIKV solely in their serum [16,18], 2 were positive exclusively in urine [17,19], 2 cases tested positive both in their serum and urine [20,21] and 1 individual tested positive for ZIKV in both his urine and serum [22]. The individual whose semen detected ZIKV showed symptoms of haematospermia, uncommon to all other infected individuals [22]. The remaining articles did not specify the method of diagnostic testing, but confirmed ZIKV in the reported cases [23,24].
Two cases in two articles, tested positive for IgM response, suggesting the symptoms were present for over seven days and virus is no longer present in the blood [2,9,15].
Travel-Associated (Vector-Borne)
A total of nine articles were reviewed reporting travel-associated transmission with high likelihood of mosquito-involved transmission. As shown in [Table/Fig-2], between the nine articles, a cumulative of 2453 ZIKV cases reported having lived in or recently traveled to a country of ZIKV transmission. All cases had confirmed ZIKV virus except for five who had pending results [16,25]. Symptomatic cases commonly demonstrated a variety of symptoms including: fever, rash, myalgia, conjunctivitis, arthralgia, and fatigue. Vector-borne transmission represents almost 95% of the cases in our systemic review.
Blood Transfusion
Only one article was found regarding transmission of ZIKV through blood transfusion dating March 2015 [Table/Fig-2]. A 55-year-old man with hepatic cancer received donated pool platelet concentrate during a liver transplant. Patient was asymptomatic, however was tested as protocol for recipients of blood transfusions. Patient tested positive for ZIKV and it is suspected to have been transmitted via the donor’s blood [30].
Intrauterine Mother to Foetus Vertical Transmission of ZIKV
Intrauterine vertical transmission of ZIKV from the mother to the foetus was examined in the seven recent studies. The pregnant women selected for the studies exhibited a characteristic ZIKV rash and other ZIKV associated symptoms such as fever, myalgia, retro-ocular and musculoskeletal pain. These symptoms were the initial signs of maternal ZIKV infection which was then confirmed by ZIKV detection in serum, urine, amniotic fluid or breast milk and ZIKV RNA sequencing. Most studies (as seen in [Table/Fig-2]) demonstrated similar foetal and newborn defects such as microcephaly, brain atrophy and calcifications, ventriculomegaly, hemisphere asymmetry and poor growth and development [6,7,26-28]. The study by Besnard M et al., showed that there was one newborn that remained asymptomatic despite containing ZIKV RNA in its serum and having a mother who was also positive for ZIKV and demonstrating symptoms of ZIKV infection during the time of delivery [13]. The only study that clearly demonstrated no microcephaly and normal foetal body circumferences was the study by Perez S et al., [29]. The cohort study performed by Brasil P et al., lost a significant amount of further screening data due to some women refusing continuing screening. Despite the reduction in patients, the 12 women remaining with abnormal ultrasonographic exams revealed that foetuses exhibited different combinations of intrauterine growth restriction, cerebral calcifications, CNS modifications, oligohydramnios and any hydramnios, abnormal arterial flow in cerebral or umbilical arteries. In this study, there were also two foetal deaths [7].
Breast Feeding Transmission
ZIKV transmission through breast feeding was tested in two studies, although definite transmission remained ambiguous [Table/Fig-2]. In both studies by Besnard M et al., and Dupont-Rouzeyrol M et al., both mothers were positive for ZIKV in breast milk and serum [13,31]. Dupont-Rouzeyrol M et al., were unable to confirm if the newborn serum contained ZIKV [31]. In Besnard’s study, both newborns had a similar serum ZIKV viral RNA load. Also, this study determined that although ZIKV RNA was present in breast milk, no replicative ZIKV was found [13].
Discussion
Through this systematic review, ZIKV has been shown to have several possible modes of transmission including sexual transmission, vector-borne transmission, blood transfusion, intrauterine mother to foetus vertical transmission and through breast milk to newborns. ZIKV is predominantly a vector-borne virus, followed by a high incidence of intrauterine vertical transmission.
The strength of this paper is the evaluation of various methods of transmission and the integration of the information found between each mode. This review aids in investigating and contextualising the recent ZIKV research in order to determine future research directions. The systematic review will help to guide health professionals and researchers with further research topics to explore. Also, it will allow people, especially those travelling to areas affected by ZIKV, and pregnant mothers to take precaution and to be proactively tested for ZIKV.
This systematic review provides important details which must be kept in mind when approaching ZIKV testing. Firstly, not all cases present with clinical symptoms. Basis of testing should not solely be focused on clinical presentation, but also on their history of recent travel to ZIKV endemic areas or exposure to persons returning from ZIKV endemic areas. Secondly, as previously mentioned, infected persons may exhibit ZIKV in their blood, urine and/or semen, otherwise IgM antibody will be detected [2,15]. ZIKV in blood has been reported to be strongly detected within the first week of transmission, however with time, viraemia lessens. This has significance with approaching testing of ZIKV as it is important for practitioners and researchers to test several bodily fluids in order to prevent misdiagnosis, propelling continuous transmission.
As several cases presented with ZIKV in urine and/or semen, while absent in the blood, this insinuates that ZIKV may replicate in other systems within the body [17,19,20,22]. Further research is needed in order to gain knowledge on ZIKV replication and its duration in various bodily fluids and systems. To be specific, knowing the duration which ZIKV replicates within the semen would allow for better prevention, as individuals may have a specific time frame to refrain from sexual intercourse in order to avoid further transmission.
Additionally, ZIKV has been shown to be highly correlated with foetal abnormalities such as microcephaly and other combinations of CNS malformations such as brain calcifications, ventriculomegaly and hemisphere asymmetry [6,7,26,27,29]. Most pregnancies in this data analysis did not come to term because of foetal death or due to mother’s choice to terminate the pregnancy as a result of a poor medical prognosis [6,7,28,29]. This indicates that ZIKV is teratogenic because of its ability to cause birth defects and often lethal effects on the foetus. The mechanism by which ZIKV operates to cause such birth defects is still unknown. It is unclear at what stage of development ZIKV infection is most dangerous and lethal to the foetus. Also, it is unknown whether passive immunity to ZIKV will be transmitted to the foetus when IgM antibody for ZIKV is present in the mother’s blood as a response to ZIKV.
Many questions and uncertainties are still present about the mechanism, mode of transmission and effects of ZIKV. Current research studies have still left many questions unanswered such as which specific mosquito species can act as vectors, the efficacy of bodily fluid transmission such as breast milk, saliva, urine, and blood, whether ZIKV reinfection is possible, if a long lasting memory immune response is possible after the first ZIKV infection, the exact mechanism of ZIKV operation to cause microcephaly and other birth defects [32] and whether maternal ZIKV antibodies can be transferred to the foetus. These are all potential future research questions that can increase our knowledge of ZIKV.
Prevention
Currently, there are several research studies working on developing multiple products to prevent and treat ZIKV. As of March 2016, 18 vaccines were in research stages of development but not tested on humans [32]. Different vaccine approaches are being studied such as inactivated virus, nucleic acid vaccines, live vector and live recombinant vaccines and more [32].
Since a vaccine is still being developed, other prevention practices should be followed such as reducing mosquito breeding sites, reducing contact between humans and mosquitoes by using mosquito nets and screens, insect repellent, light clothing and emptying containers with water [33]. Also, there have been no reported cases of ZIKV transmission through sexual contact while using condoms. Therefore, use of condoms during sex is recommended to potentially decrease sexual transmission. Also, men should refrain from having sex with pregnant women to reduce the chances of transmission to the women and the foetus [16]. ZIKV is often initially asymptomatic thus residents of ZIKV infected areas and travelers should be frequently tested for ZIKV in order to prevent its spread. Travelers should also be aware of ZIKV endemic areas and take the appropriate precautions to prevent ZIKV infection and spread [2].
Limitation
The weakness of the review was that our results need continuous update due to the constant evolving ZIKV, requiring new searches. Additionally, this further affected information provided in case reports as ZIKV laboratory confirmation was pending at times and exact infection date was unknown.
Conclusion
The ZIKV virus remains as a global concern due to its rapid spread throughout different countries. It is important to study and understand how ZIKV is transmitted in order to understand and discover different ways to prevent this global transmission. This systematic review of ZIKV transmission has allowed a comparison of different cases in different countries. ZIKV has been shown to be spread through vector-borne transmission, sexual transmission, blood transfusion, intrauterine transmission and possibly breast milk. Further research is needed in order to further understand ZIKV and how it can be prevented.