AD is a disorder with dementia that usually causes conditions such as memory loss along with thinking and behaviour problems. Its prevalence is estimated to be around 15 million worldwide. The symptoms of AD develop slowly but usually get worse over time to become severe, interfering with daily tasks [1].
AD is usually associated with degeneration of cholinergic neurons in the basal forebrain along with loss of cholinergic neurotransmission in nucleus basalis magnocellularis, amygdale, cerebral cortex, and other areas which significantly contribute to deterioration of cognitive functions [2].
Previous studies have shown that, both anticholinergic drugs and lesions of the NBM disrupt learning or memory in AD animal models in passive avoidance test [3].
Cholinergic drugs effective in AD have been reported to increase the regional cerebral blood flow of acetylcholine in the damaged brain regions [4].
AChE is the key enzyme in brain which metabolizes acetylcholine. One method for increasing the level of acetylcholine in the brain is blocking the activity of AChE [5]. The cholinesterase inhibitors such as Donepezil, Galantamine and Rivastigmine are the current approved drugs for the treatment of AD [5]. The cholinesterase inhibitors have some problems such as hepatotoxicity, short half-lives and the side effects caused by the activation of peripheral cholinergic systems [6,7].
Natural products and their components have shown promising results in management of various conditions [4,8,10] including cognition-enhancement [11]. Novel AChE inhibitors from plant sources could be valuable alternatives for treatment of AD.
Reduced forms of oxygen are produced in the brain during cellular respiration. This increase in production of free radicals has been reported to cause damage to cell membranes, enzymes, DNA, lipids, and proteins, impairing their function [14-16]. Oxidative stress is a disparity between the rates of free radical production and elimination through endogenous antioxidant mechanisms. Oxidative stress causes increase in various diseases [17-20].
The chards leaves extract has great AChE inhibitory and antioxidant activities indicating useful effect in prevention of neurodegenerative diseases such as Alzheimer [21].
The present study evaluated the effects of hydroalchoholic extract of chard leaves on learning and memory functions in AD rat model with memory deficits and intact rats.
Materials and Methods
In this experimental study was done in two months (November 2015- December 2015). All experiments were executed in accordance with the Guide for the Care and Use at Laboratory Animals and were approved by Research and Ethics Committee at Shahrekord University of Medical Sciences, Shahrekord, Iran. Male Wistar healthy rats (150-250 g) were purchased from Pasteur institution (Tehran, Iran) and housed in groups of four in steel cages at 25°C with controlled 12 hour light-dark cycle with freely available food and water.
Total of 49 male Wistar rats were divided into seven equal groups as follows:
Group 1: Control group without surgery, received distilled water via i.p injection for 15 days consecutively. (This group required for compare with AD group and confirmation lesion of NBM).
Group 2: AD group (bilateral NBM lesion, received water via i.p injection)
(This group required for confirmation lesion of NBM)
Group 3: AD + BVL groups (rats with NBM lesion received BVL extract 100 mg/kg b.w, respectively i.p for 15 days)
Group 4: AD + BVL groups (rats with NBM lesion received BVL extract 200 mg/kg b.w, respectively i.p for 15 days)
Group 5: Sham group (electrode was lowered into the NBM without passing any current, received water)
Group 6: Healthy groups without any surgery received BVL extract 100 mg/kg, respectively i.p for 15 days.
Group 7: Healthy groups without any surgery received BVL extract 200 mg/kg, respectively i.p for 15 days.
Plant Material and Preparation of the Extract: The leaves of chard were collected from a farm in Shahrekord were authenticated by a botanist in Medical Plants Research Center in Shahrekord University of Medical Sciences, Iran. The leaves were dried in the shade at room temperature and stored in a dark and cold room until use. The dried leaves of chard were soaked in ethanol and distilled water (70%) at room temperature for two days. The extract was then filtered and evaporated to dryness under reduced pressure and controlled temperature in a rotary evaporator. The extract was used when needed [11].
Stereotaxic Surgery: For stereotaxic surgery, rats were anesthetized by intraperitoneal injection of ketamine hydrochloride 110 mg/kg and Xylazine, 4 mg/kg then they were placed in stereotaxic apparatus.
They were then placed in a stereotaxic frame, and animal head was firmly positioned within the stereotaxic frame by inserting ear bars into the external ears. Following these steps, lubricating eye ointment was applied to prevent drying of the eyes. All the rats were implanted with a twisted bipolar stainless steel electrode (Plastic Products MS 301/1, 0.25 mm in diameter; Bilaney, Düsseldorf, Germany) in one hemisphere under conventional stereotaxic procedures. The electrodes were aimed to the NBM use in an incisor bar set at -2.7 mm below the interaural line. Based on the stereotaxic atlas of AP: the probe was designed at -1.30 mm from bregma, L: ± 2.8 mm both respect to bregma, and DV: -8.00 mm from cranium surface. In this procedure the NBM lesion was made for 15 s by electrolysis using a current intensity of 2 mA. After induction of lesion at each side the electrode was withdrawn. The sham operated groups underwent similar surgical procedures without delivery of current. The incision was cleaned and sutured [22].
Water Maze Test: The water maze used was a circular steel pool filled with milky water which was kept at about 24°C. A platform was submerged in one of the quartes below the surface of the water there was a training trial in which the rat was placed in the water and allowed it to remain on the platform for 10 seconds. Then it was returned to the home cage. If a rat was not able to find the platform within 60 seconds, it was placed on it and all period to remain there for 10 seconds. Each rat was given four trials daily for four consecutive days. However, the each rat was individually subjected to a probe trial session on the fifth day following removing the platform and allowing the rat to swim for 120 seconds to search for platform [7].
Passive Avoidance Test: The used passive avoidance box had one illuminated and one dark compartments equipped with a grid floor. There was a training trial in which each rat was placed in the illuminated compartment. When the rat entered the dark compartment, the door was closed and an inescapable shock was applied to the rat. One day after the training trial, the testing trial was given for this, the rats were again placed in the illuminated compartment and the time spent to re-entered the dark compartment was recorded (the step-through latency maximum testing limit was 60 second) [4].
Ferric Reducing/Antioxidant Power (FRAP) Assay: The end of behavioural tests blood samples were obtained through cardiac puncture for biochemical estimations, and then the serum and plasma were immediately separated from the blood samples by centrifugation. Antioxidant power of plasma was determined by measuring its ability to reduce Fe3+ to Fe2+ with FRAP test. FeSO4 (100 – 1000 μm concentration range) was used as a standard in FRAP assay. The results are expressed in μm [16].
Measurement of Plasma Malondialdehyde (MDA): The plasma MDA level was measured as Lipid Peroxidation (LPO) by the Thiobarbituric Acid Reactive Substances (TBARS) method. Briefly, to 100 μl plasma or standard, 100 μl sodium dodecyl sulfate (8.1%) and 2.5 ml TBA/buffer (prepared by dissolving of 0.53% TBA in 20% acetic acid as adjusted to pH 3.5 with NaOH) were added. The tubes were covered with caps and incubated at 95°C for 60 minute. The reaction was stopped by placing tubes on ice followed by centrifugation at 4000 rpm for 10 minute to separate two phases. The supernatant (20 μl) was injected into the High-Performance Liquid Chromatograph (HPLC) system.
Chromatographic procedure was done on a HPLC which was equipped with a UV absorbance detector and a 1100 series pump. An HP 3395 integrator was used to record the chromatograms, retention times and peak heights. The used colom was a technopak 10u C18 reversed-phase (emission553 and excitation 515) [23,24].
MDA Levels of Brain Tissue: Following the behavioural tests, all rats were sacrificed. The brains were quickly removed and were washed twice with cold saline solution; the whole brain was placed into glass bottles, labeled, and stored in a deep freeze (−80 °C) until processing. Tissues were homogenized in ice-cold Tris–HCl buffer (50 ml, pH 7.4) for two minutes at 5000 rpm. The homogenized solution was then centrifuged for 60 minutes at 5000 g. MDA level was then measured.
LPO was evaluated by measuring the TBARS content according to the TBA test method with slight modification. The MDA level was determined by a previously described method in which the reaction with TBA at 90–100 C° is the dominant base. The reaction in this experiment was performed for 15 minute at pH 2–3 in 90°C. The specimen was mixed with 2 volumes of 10% (w/v) trichloroacetic acid in order to precipitate the proteins. The resultant precipitate was pelleted by centrifugation. The aliquot of the supernatant was then reacted with the same volume of 0.67% (w/v) TBA in a boiling water bath for 10 minutes then it was left to get cold and absorbance was read at 532 nm using spectrophotometer [18].
DPPH Radical Scavenging Activity: Antioxidant capacity of the leaves of chard extract was measured using the DPPH assay based on the scavenging ability to 2, 2-Diphenyl-1-picrylhydrazyl (DPPH) stable radical [25]. Butylated hydroxytoluene (BHT) was used as a positive control. The samples in different concentrations (10,20,50,100,250) mg/kg were mixed with DPPH solution and ethanol. After vortexing, the tubes were left in the dark room temperature after which the absorbance was measured at 517 nm using a UV–Vis spectrophotometer (Biochrom Ltd., England). All measurements were made in triplicate and averaged. Antioxidant activities were expressed as the IC50 values. The DPPH radical scavenging activity was calculated using the following equation:
(%) = 100 × (Acontrol – Asample)/AcontrolThe concentration of antioxidant required to cause 50% reduction in the original concentration of DPPH was considered as IC50 [26].
Determination of Total Phenolic Compounds: Total phenolics in chard extract were determined with Folin–Ciocalteau reagent. Briefly, to 0.5 ml of a 5.5 g/l diluted extract, 2.5 ml of Folin–Ciocalteau reagent (diluted 10 times with water) was added. The standard curve was plotted using 12.5, 25, 50, 62.5, 100, and 125 mg/L solutions of gallic acid in methanol and water (60:40, v/v). The absorbance was measured at 760 nm. The total phenolic contents of the extracts were expressed as gallic acid equivalent (mg/g extract) [27]. The data were presented as the average of triplicate analyses.
Determination of Total Flavonoid and Flavonol Content: The amount of total flavonoids and flavonols in the chard leaves extract was determined calorimetrically as described by previously. Total flavonoids and flavonols were expressed in terms of rutin equivalent (mg/g), which is a common reference compound [28].
Statistical Analysis
All the results were expressed as mean±SE and data was analyzed in SPSS software (version 19.0) using One-way Analysis of Variance test (ANOVA) followed by post-hoc LSD test. The p<0.05 was considered statistically significant.
Results
Standardization of Card Leaves Extract: To standardize the plant extract, total phenolic, flavonoid and flavonol compounds of chard leaves extract were measured. Total phenolic compounds of chard leaves extract was 51 mg/g gallic acid equivalent that expressed as mg phenol per g of dry matter. Total flavonoid and flavonol compounds were 17.3 mg/g and 2.5 mg/g of dry matter, respectively, equivalent to Rutin (mg per g).
Radical Scavenging Activity of Beta vulgaris Extract: IC50 values for radical scavenging activity of BVL extract are shown in [Table/Fig-1].
DPPH radical scavenging activities for various concentrations of Beta vulgaris leaves extract.
| Sample | Concentration(μg/ml) | DPPH radical scavenging activity Inhibition (%) IC50 (μg/ml) |
|---|
| Beta vulgaris leaves Extract | 10 | 10.39 |
| 20 | 18.01 |
| 50 | 21.93 |
| 100 | 39.95 (IC50) |
| 250 | 81.52 |
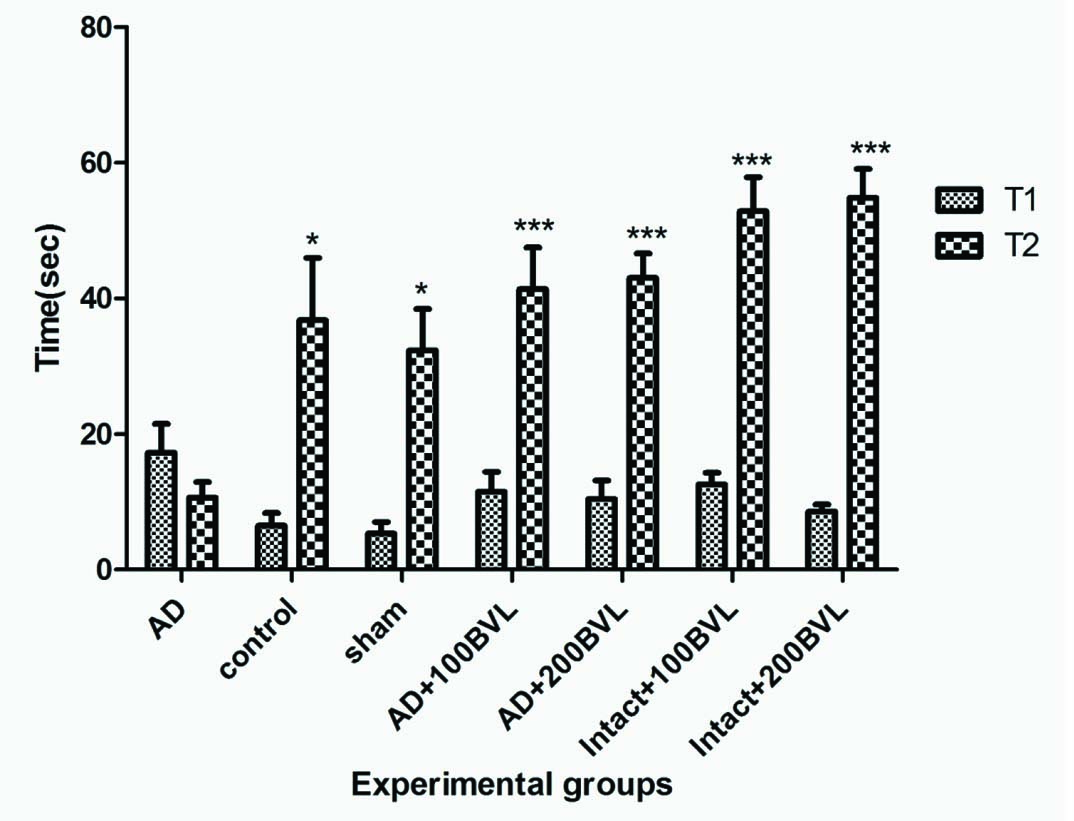
Effect of BVL extract on initial latency and step-through latency in the passive-avoidance response in rat model of Alzheimer and intact rat: Step through latency (T2) in AD group was reduced markedly in comparison to control and sham groups (p<0.05). Step through latency increased significantly in AD+100BVL, AD+200BVL, Intact+100BVL, Intact+200BVL groups when compared with AD group (p<0.05). BVL extract had no remarkable effect on initial latency in test session in experimental groups that received extract compare to AD group (p>0.05) [Table/Fig-2].
The initial latency and step-through latency in the passive avoidance response. T1: initial latency, T2: step-through latency. The data are expressed as mean ± SD; n = 7 in each group.
*p<0.05; ***p<0.01 AD versus control, sham, AD + 100 BVL, and AD+200 BVL groups (n = 7). BVL: Beta vulgaris leaves, AD: Alzheimer’s disease.
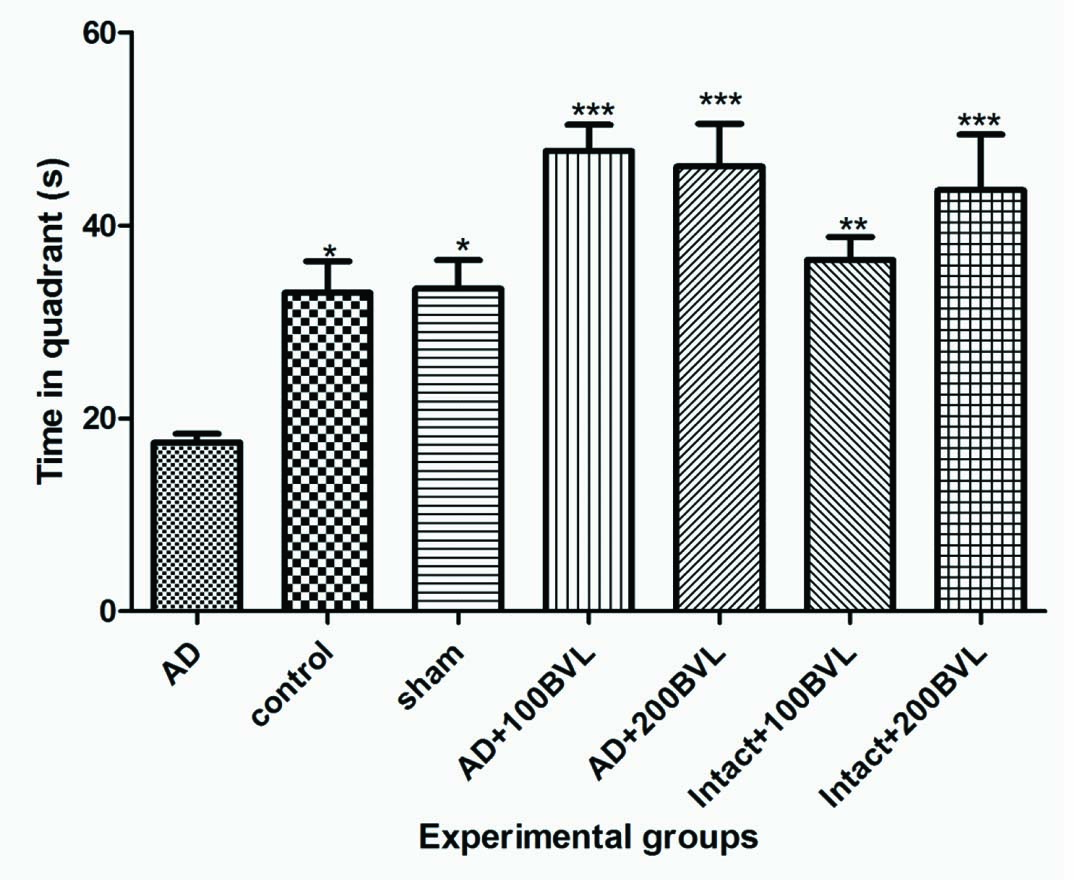
Effect of BVL Extract on Morris Water Maze Swimming Test: AD rats spent significantly less time in the correct quadrant (Zone1) compared with control group in the probe trail (p<0.05). Platform was eliminated on the probe day. In the probe test all experimental groups demonstrated a significant preference for the quadrant in which the platform was located on the preceding day (Zone 1) when compared with AD group (p<0.05). There were no significant differences between the control and sham groups in the probe test [Table/Fig-3,4].
Spent time in Zone 1 during the probe trial in experimental groups. The data are expressed as mean ± SD; n = 7 in each group.
*p<0.05; ***p<0.01
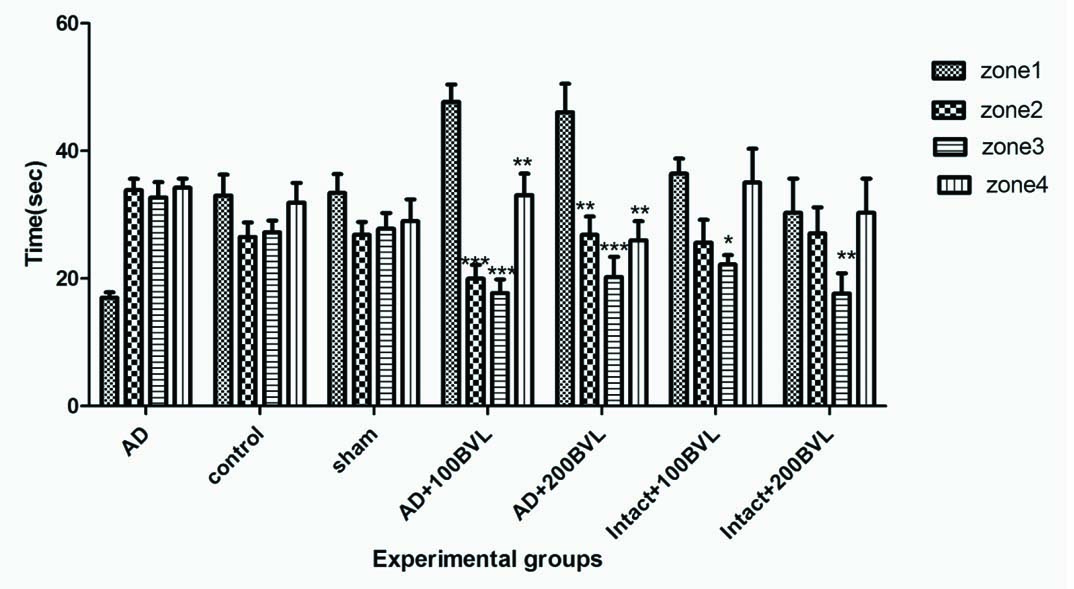
Spent time in each quadrant (from left to right: Zone 1, Zone 2, Zone 3, Zone 4 in each group) during the probe trial in experimental groups.
*p<0.05; ***p<0.01
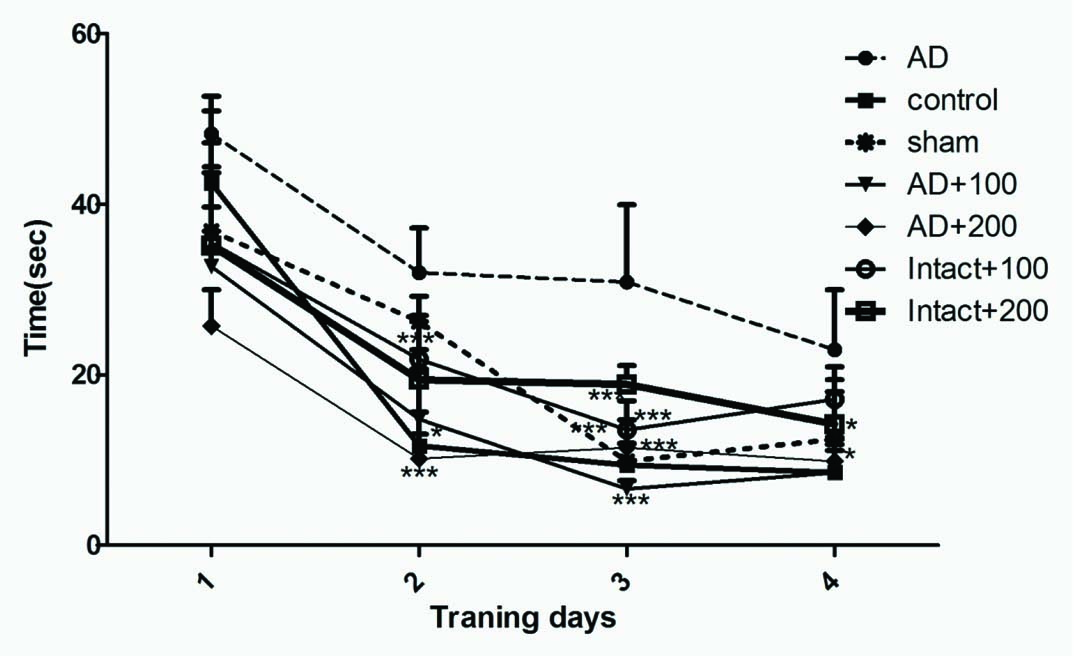
AD group showed significant increase in latency before finding the escape platform across the training days when compared with control group (p<0.05). Performance of sham rats was not significantly different from control rats. The decrease in the escape latency improved significantly on day two, three in control, AD+100BVL, AD+200BVL groups when compared with AD group (p<0.05). The latency before reaching platform on day four significantly decreased in control and AD+100BVL when compared with AD group [Table/Fig-5].
Spatial learning in a hidden platform model in experimental groups during four training days.
*p<0.05; ***p<0.01
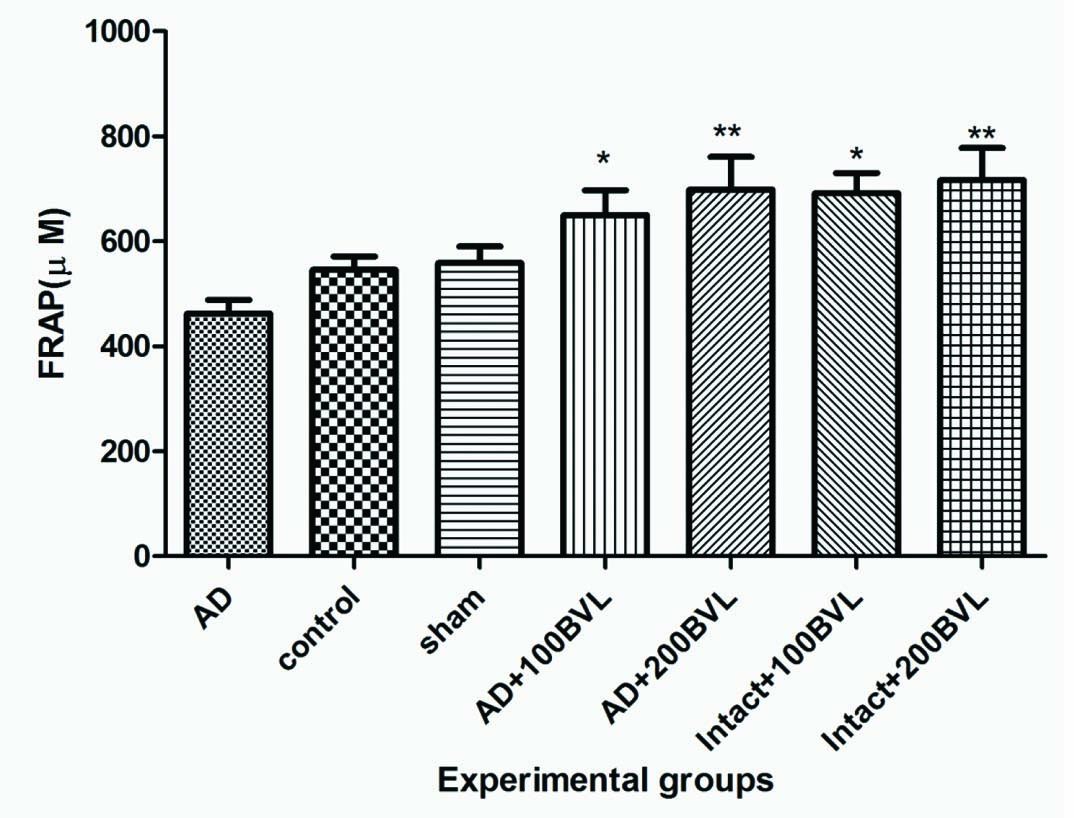
Plasma Antioxidant Level: Administration with BVL extract significantly increased plasma antioxidant levels in AD+100BVL, AD+200BVL, intact+100BVL, intact+200BVL groups as compared with AD group (p<0.05). No significant change (p>0.05) was observed in plasma level antioxidant power between control group and sham group [Table/Fig-6].
Plasma antioxidant capacity (FRAP) in experimental groups.
*p<0.05; ***p<0.01
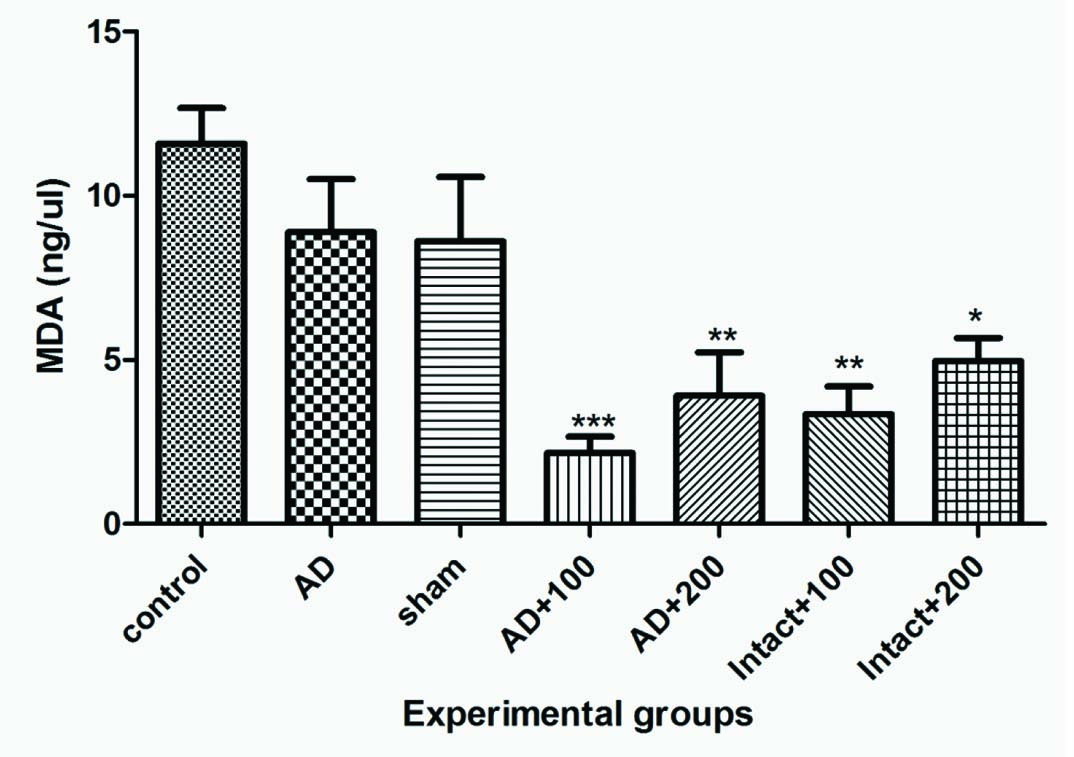
Plasma MDA Levels: In the experimental group administration with 100 and 200 mg of BVL extract in intact groups and AD groups significantly decreased the plasma MDA levels when compared with control group (p<0.05). No significant difference (p>0.05) was observed between sham and control groups [Table/Fig-7].
Plasma MDA levels in experimental groups.
*p<0.05; ***p<0.01
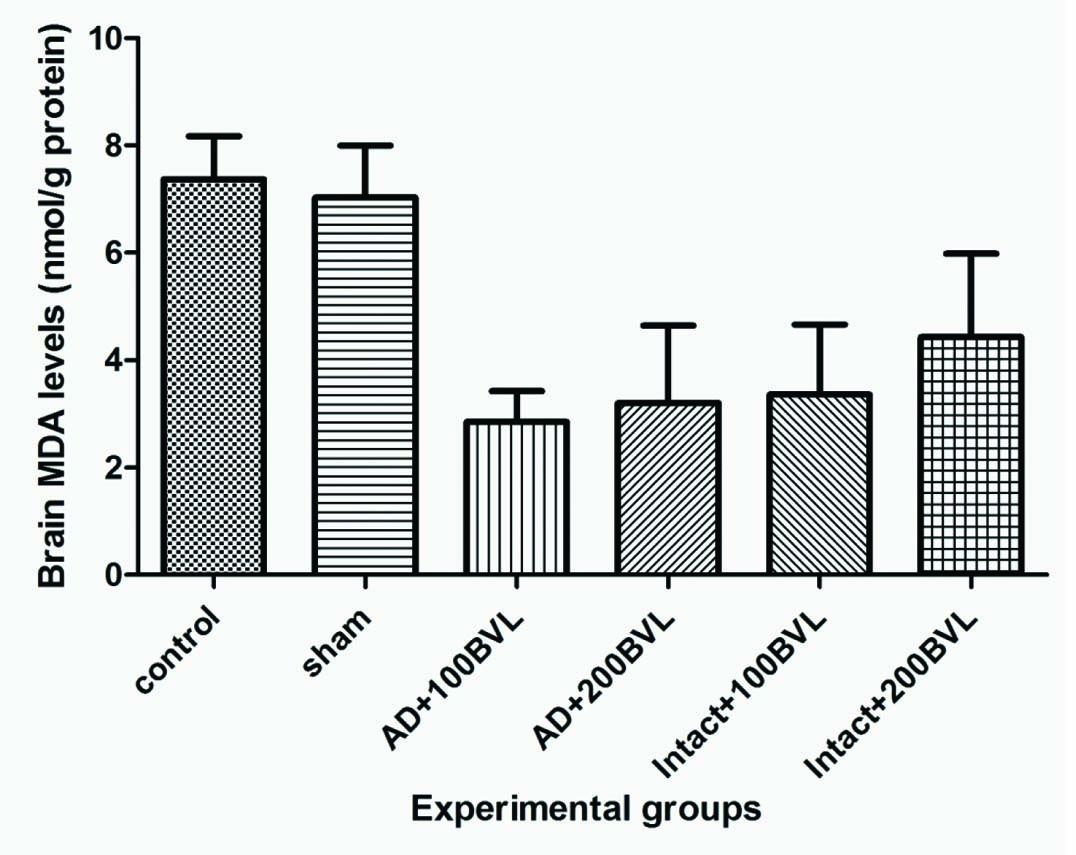
Brain MDA Levels: BVL extract reduced brain MDA levels in AD groups and intact groups that received extract but changes not significant (p>0.05) [Table/Fig-8].
Brain MDA levels in experimental groups.
*p<0.05; ***p<0.01
Discussion
In the present study, the effect of improving memory deficit of BVL extract was evaluated using the AD rat model induced by NBM lesion.
The NBM is an important brain structure involved in information processing. Studies showed that lesions of the NBM induced impairment on memory formation in various learning tasks such as passive avoidance learning and memory [24,25].
Most basal forebrain magno cellular neurons, including those in NBM, are cholinergic. The NBM provides the primary cholinergic projection to the cerebral cortex [15]. Basal forebrain system including the NBM has been widely implicated in the pathogenesis of AD and its accompanying cognitive deficits [26].
Studies have shown that the BVL extract have great AChE inhibitor activity in vitro [11].
Our results have shown that the NBM lesions have induced significant learning and memory disturbance in passive avoidance paradigm and spatial cognitive deficit in Morris water maze and shuttle box, whereas treatment of rats with BVL extract for 15 days could significantly attenuate these abnormalities.
In our study BVL extract significantly increased the step through latency in NBM-lesioned group receiving extract in shuttle box test. In the probe test all experimental groups that received extract demonstrated a significant preference for the quadrant in which the platform was located on the preceding day. Our observations suggest that effect of BVL extract on enhancing learning and memory in AD rat model may be related to mediation of the cholinergic neurotransmitter system.
Zizyphus jujube is also a potent activator of choline acetyltransferase. Its extract treatment with doses of 500 and 1000 mg/kg could significantly prevent the cognitive impairments following NBM lesion and thus suggesting the therapeutic potential of this extract in aging and age-related neurodegenerative disorders. Choline acetyltransferase activators increase the synthesis of acetylcholine to boost the endogenous levels of acetylcholine in the improvement of cognitive function [27,28].
Lesions of the septohippocampal and NBM-neocortical pathways can assess the contribution of cholinergic deficits to cognitive disorders [4].
The large lesions of the NBM can damage the globus pallidus located dorsolaterally to the NBM, which may result in deficits in passive avoidance and sensorimotor functioning [22].
A significant correlation was found between cortical choline acetyltransferase depletion after NBM lesions and several neurological parameters or performance in the Morris water maze [29].
AD is associated with inflammatory processes. Reactive oxidative species (ROS) may also be very harmful as they can readily attack biomolecules such as lipids, proteins, and DNA, leading to various disorders, e.g., inflammation, arteriosclerosis, cancer, and neurological disorders such as AD. The use of antioxidants may be useful in the treatment of AD [29].
The Beta vulgaris aqueous and ethanolic extracts have previously been shown to have antioxidant activity, decreasing the radical ions [30].
In order to elucidate the mechanism of anti-amnesic activity of BVL extract, LPO and serum antioxidant levels were measured following the shuttle box and Morris water maze test. LPO is one of the major outcomes of free radical-mediated injury to tissue. Peroxidation of fatty acyl groups, mostly in membrane phospholipids has three phases. The most abundant diffusible products of lipid peroxidation are chemically reactive aldehydes such as MDA [31,32].
The Beta vulgaris shows antioxidant activity and free radical scavenging activities which might be also due to presence of terpenoids. This property reduces LPO which may not only prevent or slow down the onset of necrosis but also improves vascularity. Beta vulgaris extract has also shown to significant inhibit oedema. The maximum activity showed during second and third hours, the results were highly significant as compared to standard [33].
Mokhtari-Dehkordi S et al., investigated the effect of methanolic extract of leaves and roots of Beta vulgaris on motor coordination in male Wistar rats. The rats which received 50 mg/kg/day of the extracts had better motor coordination than those in the control group. A 100mg/kg concentration of the extract showed no effect on motor coordination. These findings show that Beta vulgaris extract improves motor coordination through its active chemical compounds and its effects on the central nervous system [34]. Therefore, Beta vulgaris extract other than ameliorating effect on memory and learning, has several therapeutic effects which might have benefit the users.
Limitation
It should be noted that this study had some limitations. The surgery, the duration of the study and the process of the stereotaxis caused high level of mortality. Also, Beta vulgaris has AChE activity and if we were able to measure this effect in brain and serum, we would be better able to discuss about the extract mechanism of action [34].
Conclusion
BVL extract ameliorated NBM lesion-induced memory impairment in the passive avoidance and the Morris water maze tasks in rat. These results suggest that the beneficial effects of BVL extract may be related to mediation of the cholinergic nervous system.