Cigarette and tobacco usage has been considered as a peril for numerous disease outbreaks in many developed and developing countries [1]. Smoking has been established as a major risk factor of periodontal disease and has an astute effect on the prevalence, extent and severity of periodontitis [2]. It is an established fact that clinical parameters including probing pocket depth and clinical attachment loss are increased in smokers as compared to non-smokers [3].
Periodontal disease is highly progressive in smokers and their response to periodontal therapy is significantly reduced than non-smokers. However, there is reduction in bleeding on probing and inflammatory response due to plaque accumulation among smokers [3]. These signs of inflammation could be masked due to histological changes such as increased epithelial thickness and altered microvasculature of the gingival connective tissue [4,5]. Reduced vascular density and lumen area of the gingival vessels in smokers have been stated in literature [6]. Nicotine plays a major role in promoting the rate of proliferation of gingival epithelium, thereby increasing the epithelial thickness [7].
Among the different immune cells present in the periodontal tissues, mast cells have been detected both in healthy as well as in inflamed tissues. Mast cells are involved in abundant activities ranging from control of vasculature, host defense, tissue injury and repair and allergic inflammation. Mediators that are derived from these cells are stored in secretory granules and are released by degranulation or can be newly generated when mast cells are appropriately activated. The substantial contribution of mast cell mediators to tissue damage and progression of inflammatory responses make the regulation of mast cell activity vital in the management of many inflammatory diseases [8].
Huang S et al., suggested that mast cell degranulation may contribute to the progression of periodontal disease and there is strong correlation between the density of mast cells, degranulation and severity of periodontitis [8].
With the advent of digital photomicrography, computer interphasing and powerful image analysis software, the measurement procedures have been greatly simplified. This digital technology has created a major impact in the microscopic analysis of images. Image analysis is the extraction of numerical information from digital images and has its advantages over visual assessment.
The definitive reasons for the effect of smoking on epithelium and microvasculature has not been conclusive and limited attention has been given to the role of mast cells in smoking associated periodontal disease. Hence, this study was designed to assess and compare the epithelial thickness, vascular density, lumen area, and the number of mast cells in smoking and non-smoking associated chronic periodontitis using image analysis software.
Materials and Methods
Study Design
The present cross-sectional study was conducted after attainment of the Institutional Ethical Committee approval (Ethical committee reference no. 014/10), on 30 patients diagnosed with chronic periodontitis between the age group of 33 to 78 years drawn from the Departments of Periodontics and Oral and Maxillofacial Surgery, Annoor Dental College and Hospital, Muvattupuzha, Kerala, India, from March to August, 2016.
A power analysis of histological parameters under consideration at a power of 80% revealed that 14 samples for each of the two groups are required to provide an α=0.05. The total sample of 30 systemically healthy individuals diagnosed with chronic periodontitis with a probing depth of more than 5 mm, clinical attachment loss of more than 3 mm and minimum single tooth indicated for extraction due to poor periodontal prognosis were categorized into two groups of 15 each. Patients who smoked ≥10 cigarettes/day for >3 years were considered under the test group and with no history of smoking under the control group. Informed consent was from all participants included in the study and the smoking status was assessed by questionnaire filled by the patient. Patients with systemic diseases that influence the progression of periodontitis and those under medications with adverse effects on the periodontium were excluded from the study.
Sample Collection and Tissue Preparation
The gingival biopsy was obtained from patients undergoing extraction due to poor periodontal status of the involved tooth. Incisional biopsies of approximately 5 mm thickness were attained with a scalpel blade (no. 15) from suitable sites at the time of extraction. The biopsied specimens were fixed in N/10 formalin solution, processed, embedded in fresh paraffin wax and serially sectioned at 4 μm thickness. Two sections were obtained and stained with H&E and toluidine blue respectively. Sections stained with H&E were used to assess the epithelial thickness and microvasculature and sections stained with toluidine blue were used to assess the number of mast cells. The slides were analyzed using Binocular research light microscope (Digiscope with CCTV) (Lawrence & Mayo) LM-52-1712 “LYNX” Digiscope at 100X and 400X magnifications. Series of images were captured using a Complementry metal-oxide-semiconductor (CMOS) camera using Network Management Software (NMS). The images were analyzed and assessed using ImageJ analysis software (Image Proplus-version 4.1.0.0) for Windows 95/WT/98 after accurate calibration with the standardized microscopic profile tool as per the software.
Assessment of Epithelial Thickness
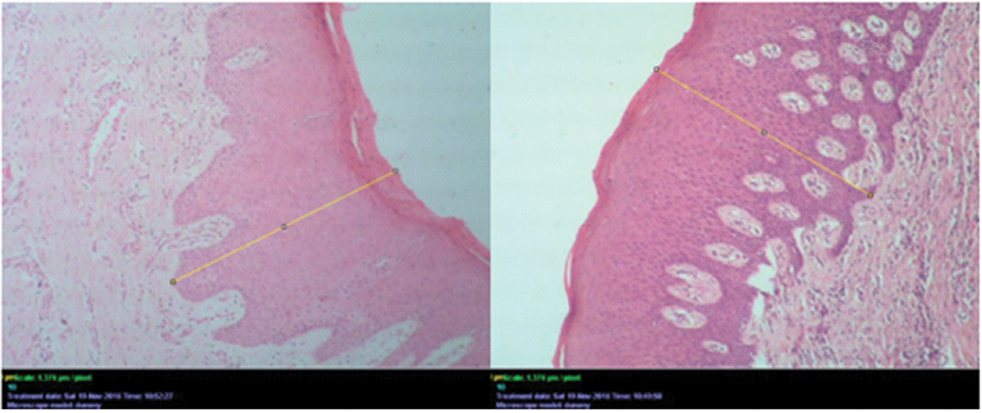
The images for assessing the epithelial thickness were captured under 100X magnification from three different areas. Epithelial thickness was measured from the surface of epithelium to the basement membrane in μm using straight line measuring tool of the software, in a chosen field and the average obtained was considered as the epithelial thickness per slide [Table/Fig-1].
H&E stained slide for measurement of epithelial thickness using line tool in ImageJ analysis software (100X).
Assessment of Microvasculature
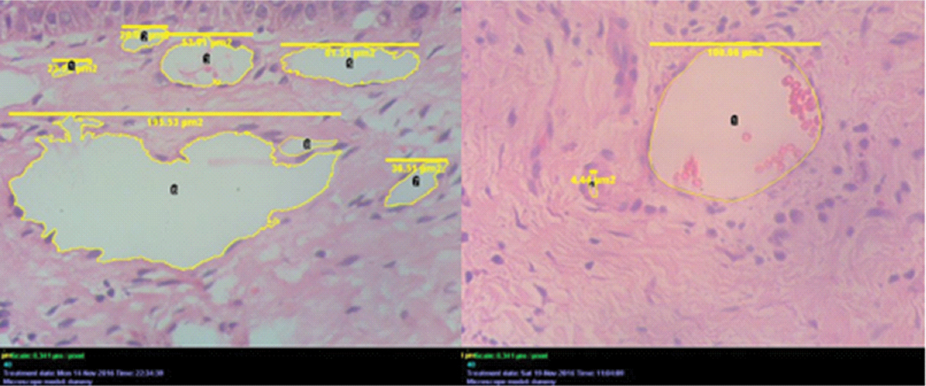
The images for assessing the microvasculature were captured under 400X magnification. Total number of blood vessels were counted using the cell counter tool in the ImageJ analysis software. The lumen areas were assessed using a cell outliner tool available under plugins in the toolbar of the software. Based on lumen area, vessels were classified into three categories as small (≤33 μm2), medium (34-64 μm2) and large (≥67 μm2) [9] [Table/Fig-2].
H&E stained slide for calibration of number of blood vessels and lumen area of blood vessels using cell counter tool in ImageJ analysis software (400X).
Assessment of mast cells
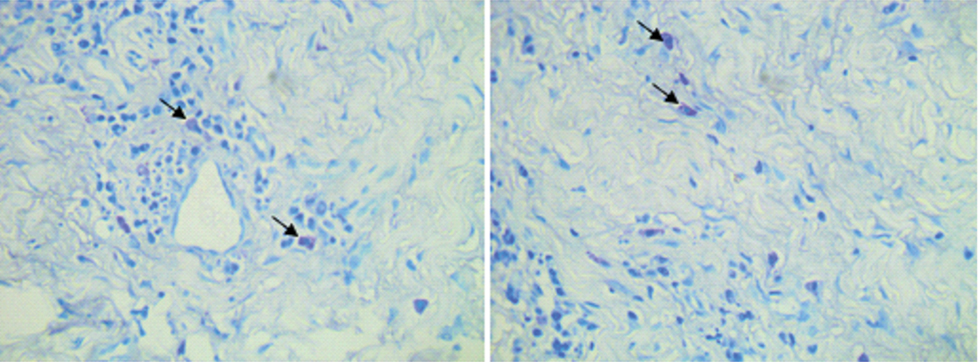
Series of images of 25 consecutive microscopic high power (400X) fields were randomly captured and assessed for the number of positively stained mast cells in the periodontal tissues [Table/Fig-3]. Scoring of mast cells was done using the cell counting tool of the software and frequencies of mast cells were tabulated for further statistical analysis.
Toluidine blue stained slide with magnification 400X; Mast cells stained purple in color, arrows indicating the mast cells.
Statistical Analysis
Independent sample t-test was used to compare the epithelial thickness, vascular density, lumen area and mast cells in smokers and non-smokers. p<0.05 was considered to be statistically significant. Data was analyzed using Statistical Package for Social Sciences (SPSS) version 22.0.0.0.
Results
Out of total 30 patients, the study comprised of 12 females and 18 males.
The mean epithelial thickness among smokers was increased (468.924±105.217 μm) when compared to non-smokers (384.278±120.915 μm) and showed statistically significant difference [Table/Fig-4].
Mean comparison of epithelial thickness, number of blood vessels and area of lumen and number of mast cells.
| Parameter | Smoker | Non-smoker | p-value |
|---|
| Epithelial thickness (μm) | 468.924±105.217 | 384.278±120.915 | 0.047* |
| Vascular density (μm2) | 18.270±10.546 | 17.380±7.839 | 0.790 |
| Area of lumen (μm2) | 6.089±3.515 | 5.792±2.613 | 0.790 |
| No of mast cells (cells/μm2) | 35.530±27.926 | 15.560±17.877 | 0.024* |
Test used: Independent sample t-test; p<0.05 - statistically significant; * Statistically Significant
The mean number of blood vessels/μm2 did not show any statistically significant difference in smokers (18.270±10.546 μm2) and non-smokers (17.380±7.839 μm2) . The mean area of lumen of blood vessels was higher in smokers (6.089±3.515 μm2) than in non-smoker (5.792±2.613 μm2) but it was not statistically significant. The mean density of mast cells were increased in smokers (35.530±27.926 cells/μm2) with statistically significant difference when compared with non-smokers (15.560±17.877 cells/μm2) [Table/Fig-4].
The mean value of number of small blood vessels (smokers- 15.400±11.419; non-smokers-13.500±8.367) and large blood vessels (smokers-1.400±1.549; non-smokers-0.938±0.998) was higher for smokers compared to non-smokers and it was also not found statistically significant. But the mean value of number of medium blood vessels was higher for non-smoker (2.938±2.144) than smokers (1.467±1.302) and it was statistically significant [Table/Fig-5].
Comparison between small, medium and large blood vessels.
| Parameter | Small | Medium | Large |
|---|
| Smoker | 15.400±11.419 | 1.467±1.302 | 1.400±1.549 |
| Non smoker | 13.500±8.367 | 2.938±2.144 | 0.938±0.998 |
| p value | .600 | .030* | .328 |
Test used: Independent sample t-test; p<0.05 - statistically significant; * Statistically Significant
Discussion
Smoking has been implicated to be a major risk factor of periodontal disease extending its deleterious effects clinically, histologically and prognostically. It has been associated with greater pocket depth, attachment loss, furcation involvement and alveolar bone loss [10]. Smokers demonstrate reduced inflammatory clinical signs that might be attributed to changes in gingival microvasculature and increased gingival epithelial thickness [6,7]. The by-products from tobacco oxidation produce peripheral vasoconstriction or damage the vasculature thereby reducing gingival bleeding, redness and edema and affect the progression of periodontal diseases [11,12].
Investigators have projected the influence of nicotine on histamine released by mast cells, the role of mast cell and its constituents in periodontal tissue breakdown [13]. Myint M et al., in their study found number of mast cells equal to and often out numbering macrophages in inflamed periodontal disease [14].
Previous studies have been evaluated the association of gingival epithelial thickness and microvasculature to periodontitis and smokers, however the relation of mast cells to above parameters have yet not been elucidated [4-10]. Considering the above facts, the current histomorphometric study was performed to evaluate and compare the changes occurring in the gingival epithelium, microvasculature and mast cell population between smokers and non-smokers in periodontitis. The age of the subjects in the study has been matched as gingival vasculature and age has been associated [15].
The current study projected an increased thickness of gingival epithelium of smokers when compared to non-smokers with chronic periodontitis and the values were statistically significant. This was in accordance with the study by Villar CC and de Lima AF who observed greater epithelial thickness in smokers independent of the gingival health [5].
Other similar studies by Saebi KH et al., [16], Seyedmajidi M et al., [17], Prakash P et al., [11], Bajagic V et al., [10], Kumar and Faizuddin [9], and Scardina GA and Messina P [4], also established an increased epithelial thickness in smokers. Our study was contradictory to the findings of Naderi NJ et al., [18], that did not show any difference in the thickness of epithelium between smokers and non-smokers in patients with chronic periodontitis and this could be ascribed to the difference in the study design and technique used in assessing the epithelial thickness.
Controversial to the above verity, the increased epithelial thickness in smokers in our study could be attributed to the fact that increase in local temperature and by-products from tobacco oxidation induced an increase in gingival epithelial thickness [4]. This fact was also confirmed by the studies of Gultekin SE et al., that nicotine increased the rate of proliferation of gingival epithelium [7].
The current study also revealed an increased vascular density in smokers than non-smokers but was found to be statistically insignificant. The mean value of number of small blood vessels and large blood vessels and the mean area of lumen of blood vessels was higher for smokers compared to non-smokers but statistically not significant. The results of this study were in agreement with the results of Mirbod SM et al., [19].
The present study was contrary to the studies by Sonmez S et al., [20], Sreedevi M et al., [21], Scardina GA and Messina P [4], Seyedmajidi M et al., [17], with regard to the vascularity. Kumar V and Faizuddin M also reported no significant difference between vascularity of smokers, (12.388±6.472 μm2) and non-smokers (14.800±4.901 μm2) this could be due to the increase in the proportion of smaller blood vessels in smokers (83.9%) and in non-smokers (82.9%), mean area of lumen among smokers is 19.290±8.775 μm2 and in non-smokers is 20.044±7.896 μm2 but the results were not statistically significant [9].
In the present investigation the mean values for large blood vessels was higher for smokers and medium blood vessels higher for non-smokers. This variation could be due to vascular changes that may occur in long standing chronic inflammation, remodeling of the gingival plexus to prominent loops running perpendicular to the epithelial surface [19]. As the biopsy samples taken for this study were from the chronically inflamed gingiva, might be a reason for the difference.
This study also suggested that vascular density is unaffected by smoking; however, smokers showed a higher percentage of smaller blood vessels. This could be due to the fact that nicotine up-regulates Vascular Endothelial Growth Factor (VEGF) and their receptors, promoting neoangiogenesis [22]. Presence of a higher proportion of smaller blood vessels in smokers may be one of the reason for the suppression of inflammatory signs in smoking population. Shimazaki Y et al., reported low expression of Soluble Intercellular Adhesion Molecule-1 (sICAM-1), a marker of vascular endothelial dysfunction, in the Gingival Crevicular Fluid (GCF) which could contribute to decreased bleeding in smokers [23]. Reduced capillary diameter and density of blood vessels in the gingival tissues of smokers explains the reduction of gingival index scores as well as clinical changes of reduced redness and bleeding in smokers.
Mast cells originate from pluripotent hematopoietic cells in the bone marrow, after partial differentiation they enter the peripheral mucosal or connective tissue microenvironments and complete their differentiation [24]. Gingiva contains the highest number of mast cells, dispersed throughout gingival connective tissue, closely associated with endothelial cells and are located either subepithelially and/or intra epithelially. Gingival mast cells are sensitive to chemical substances, physical agents, like low intensity laser irradiation or metal ions that induces considerable degranulation [25].
Studies by Batista AC et al., [26], Vahabi S et al., [27], Agrawal R et al., [28], Lagdive SS et al., [29], suggested that mast cell counts may be increased in periodontitis. These findings indicate the role of mast cells in chronic periodontal tissue breakdown. Histamine released by mast cells breaks down the tissue barrier, cause edema and helps cellular infiltration. The Matrix Metalloproteinases (MMPs) 1,2 and 8 expressed by mast cells play a vital role in the degradation of the major components in extracellular matrices. Additionally, tryptase have the property to cleave component of collagen and activate latent collagenase that participates in tissue damage in periodontitis [30].
In the present study the number of mast cell was significantly increased in smokers as compared to the non-smoking group. The effect of periodontal disease pathogenesis combined with the changes induced by smoking must have resulted in the increased mast cell density. This is consistent with the increased periodontal destruction seen in smokers.
Studies by Gemmell E et al., was contradictory with decreased mast cells in inflamed sites [31]. This can be ascribed to mast cell degranulation. When subjected to injurious agents the mast cells lose their integrity, release their granules thereby losing their visibility under light microscope.
Based on the present study, increased mast cells has called attention with respect to the possible participation of mast cells in the destructive events and defense mechanism both as effector and responsive cell in chronic inflammation, as well as the possible functional relationship between mast cell and immunocompetent cell populations in periodontal lesions [26].
Limitation
In this study, the assessment of epithelial thickness was done by measuring the total distance from the basal layer to the surface of the epithelium, the thickness of different layers such as the outer and inner epithelium, keratinous layer and the basement layer were not assessed separately. This could have given an insight about which layer was more affected by smoking resulting in the thickness of the epithelium. Furthermore, immunohistochemistry for identifying markers for epithelial and connective tissue breakdown would have given a direct correlation with the cellular alteration and association with smoking. However if the inflammatory and bleeding index were also taken into account, a better understanding about the association between the clinical signs and histological findings could have been assessed. The assessment of the difference in the distribution, size and degranulation of the mast cells in both the groups would have given a better insight about the role of mast cells in smoking.
Conclusion
Smoking plays a direct role in the pathogenesis and progression of periodontitis and a direct correlation between smoking, gingival epithelium and mast cell number in chronic periodontitis has been elucidated. Mast cells have an additive profound effect on periodontal tissue destruction. The association, interrelation and the underlying molecular mechanism between the frequency of mast cells and their further influence on the vasculature or the epithelial thickness has yet to be elucidated. Further studies with more sample size utilizing advanced histopathological diagnostic methods are needed to confirm the results of this study. In addition, smoking cessation can be considered as one way to revert the harmful effects of tobacco on periodontal risk.
Test used: Independent sample t-test; p<0.05 - statistically significant; * Statistically Significant
Test used: Independent sample t-test; p<0.05 - statistically significant; * Statistically Significant