Thus, this in vitro study was performed to evaluate the antibacterial efficacy of Ca(OH)2 mixed with imipenem and OCT against E. faecalis and compare it to that of Ca(OH)2 with saline using dentin block model.
Materials and Methods
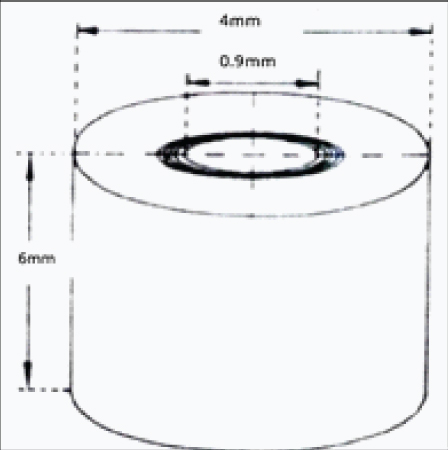
This study was an in-vitro study carried out at KLE VK Institute of Dental Sciences in collaboration with KLEs Dr. Prabhakar Kore Hospital and Research Centre, KLE University, Belagavi, Karnataka. The project was cleared by the Institutional Ethical Committee board of KLE University. The modified Haapasalo and Orstavik model was used for this study, similar to the one used by Krithikadatta J et al [16]. The extracted human mandibular first premolar teeth (n=125) were decoronated 5 mm below cemento-enamel junction (CEJ) using rotary diamond disk (DENTSPLY Tulsa, USA). Roots were sectioned to obtain 6 mm of middle third portion. Cementum was removed using cylindrical diamond bur (Mani Inc., Japan) and external diameter was standardized at 4 mm. Internal diameter of 0.9 mm was standardized using GG Drill No. 3 (Mani Inc., Japan) with a slow speed contra-angle hand piece (NSK, Tokyo, Japan) [Table/Fig-1,2]. The inorganic and organic debris were removed by treating the blocks with 17% Ethylene Diamine Tetraacetic Acid (EDTA) (Ammdent, India) for five minutes, followed by 3% sodium hypochlorite (NaOCl) (Vishal Dentocare, Gujarat, India) for five minutes. Dentin blocks were dipped in distilled water (Universal Biochemicals, India) for five minutes followed by sterilization in an autoclave (Confident, Bengaluru, India) at 121°C for 15 minutes [16,17].
Schematic view of prepared specimens.
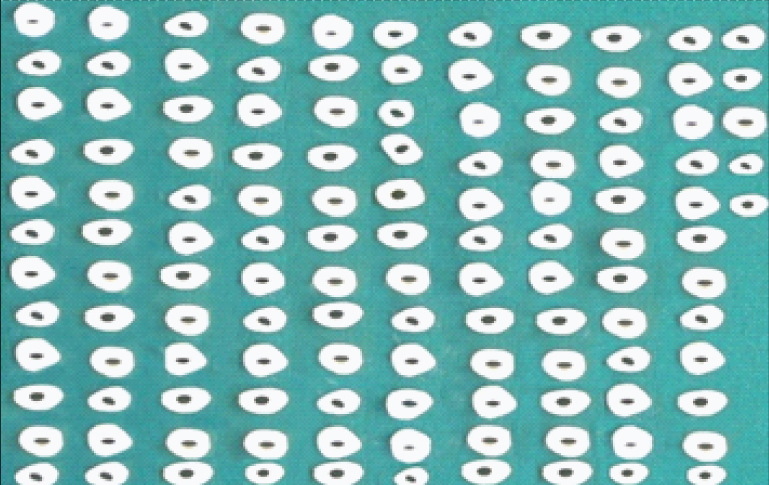
Prepared dentin specimens (n=125).
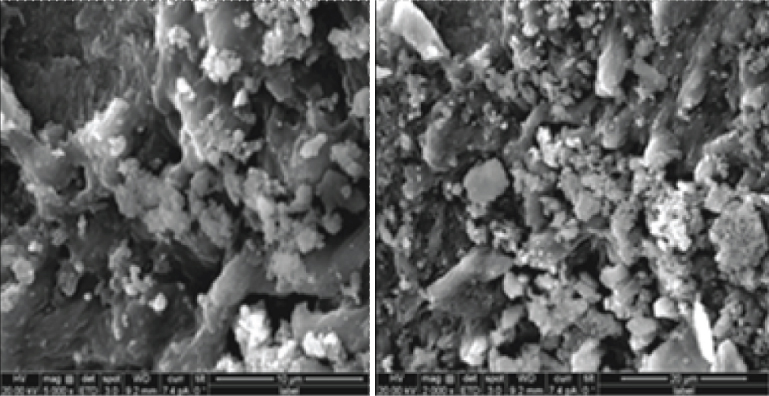
24 hour colonies of pure culture of E. faecalis (ATCC 35550, IMTECH, Chandigarh, India) grown on Brain Heart infusion (BHI) (Himedia, Mumbai, India) agar were suspended in 5 ml of BHI broth and incubated for 24 hours at 37°C in a bacteriological incubator (Labotech, BD instruments, Ambala, India). The culture suspensions were adjusted to match the turbidity equivalent of a 0.5 McFarland standard. The sterile dentin blocks were then transferred aseptically into individual microcentrifuge tubes (Tarsons, Kolkata, India). Containing 1 ml of BHI broth. About 50 μl of bacterial culture suspensions was added to each of these tubes and incubated at 37°C for 48 hours. The dentin blocks were then transferred to a fresh broth containing bacterial culture, every 2nd day upto 21st day [Table/Fig-3] [18]. All of these procedures were performed in a Vertical laminar airflow (Questt international, Bengaluru, India) to maintain aseptic conditions. The purity of the culture was confirmed by subculturing 5 μl of the broth from the incubated blocks in the respective broths, on agar plates. Five blocks were selected randomly and were used for checking the depth of penetration of microorganisms using Scanning Electron Microscopy (SEM) (FEI QUANTA 200, Hillsboro, USA) [Table/Fig-4] [19].
Infected blocks after 21 days.

SEM Images showing dentinal tubules infected with E.faecalis.
After the incubation period, the infected dentin blocks were irrigated using 5 ml of sterile saline (Baxter India Limited, Bengaluru, India), to remove the incubation broth. Two layers of nail varnish was applied to the outer surface of each specimen to prevent the contact of medicament with the external surface.
The dentin blocks were then randomly assigned to the following three groups (n=40) depending on the intracanal medicament placed inside the canal:
In Group I, the Ca(OH)2 powder (Vishal Dentocare, Gujarat, India) was mixed with sterile normal saline in the ratio of 1.5:1 wt/vol i.e.150 mg of Ca(OH)2 powder was mixed with 1 ml of sterile normal saline [17].
In Group II and III, standard minimum inhibitory concentration of 6.25 μg/ml and 15.625 μg/ml for imipenem (Lupinem, Hetero Lab, Solan, India) and OCT (Dishman Pharmaceuticals and chemicals Ltd, Ahmedabad, India) respectively, determined by the Broth dilution method were prepared in 1 ml sterile normal saline and mixed with 150 mg of Ca(OH)2 respectively.
After medicament placement, the blocks were sealed below and above using paraffin wax (Vishal Dentocare, Gujarat) followed by incubation in an aerobic environment, at 37°C [Table/Fig-5]. On 2nd and 7th day, twenty blocks were randomly selected from each group respectively and medicament was removed by irrigation with 5 ml of sterile saline [Table/Fig-6]. Next, dentin debris were harvested at a depth of 400 μm by using GG drill No. 5 [Table/Fig-7] and collected in 1 ml of sterile broth followed by incubation in aerobic environment at 37°C for 24 hours [Table/Fig-8]. After the incubation period, the contents of each microcentrifuge tube were assessed by measuring the optical density on a 96 well microtitreplate using the ELISA READER (Rapid Diagnostics, Delhi, India), at 620 nm [Table/Fig-9,10].
Blocks sealed above and below with paraffin wax.

Removal of the medicament from the block on 2nd and 7th day.
Harvesting of dentin debris using GG drill no 5.

Dentin debris collected in 1 ml of sterile BH broth.
BHI broth containing dentin debris pipetted to 96 well microtitre plate.
96 well microtitre plate containing BHI broth with dentin debris placed in the Spectrophotometer - ELISA reader.
Statistical Analysis
Two way analysis of variance (ANOVA) was used to analyze the differences among the groups. The Tukey’s multiple post hoc test was then used to compare individual groups, separately for the different intervals of time. The p value <0.05 was considered statistically significant.
Results
All the three medicaments used in this study exerted antimicrobial activity at the two tested time intervals.
At day 2, a difference was observed in the optical density values between three Groups (I, II, III) (F=2.2126, p=0.1187) at 5% level of significance. However, the difference was not statistically significant [Table/Fig-11]. It implies that, the antibacterial activity at the end of day 2 was similar. Further, pairwise comparisons were done by using Tukey’s multiple post hoc test. It revealed that all the three experimental groups exhibited antibacterial activity on the 2nd day but group II (Mean Optical Density (OD)= 0.2995) exhibited better antibacterial activity than Group I (Mean OD=0.3795) and III (Mean OD=0.3380). Also, the antibacterial efficacy of Group III was seen to be better than Group I on day 2 [Table/Fig-12].
Comparison of optical density values among the three groups (I, II, III) at the end of day 2 by One way ANOVA.
| Source of variation | Sum of squares | Degrees of freedom | Mean sum of squares | F-value | p-value |
|---|
| Between groups | 0.06 | 2 | 0.03 | 2.2126 | 0.1187 |
| Within groups | 0.82 | 57 | 0.01 |
| Total | 0.89 | 59 | |
Pairwise comparison of the optical density values of three groups (I, II, III) at two intervals (at the end of day 2 and day 7) by Tukey’s Multiple post hoc test.
| Groups with subgroups | Group I day 2 | Group I day 7 | Group II day 2 | Group II day 7 | Group III day 2 | Group III day 7 |
|---|
| Mean | 0.3795 | 0.3884 | 0.2995 | 0.2315 | 0.3380 | 0.3271 |
| SD | 0.0696 | 0.0959 | 0.1582 | 0.1413 | 0.1161 | 0.1180 |
| Group I day 2 | - | | | | | |
| Group I day 7 | p=0.9999 | - | | | | |
| Group II day 2 | p=0.2915 | p=0.1867 | - | | | |
| Group II day 7 | p=0.0023* | p=0.0010* | p=0.4745 | - | | |
| Group III day 2 | p=0.8832 | p=0.7691 | p=0.9128 | p=0.0500* | - | |
| Group III day 7 | p=0.7386 | p=0.5905 | p=0.9784 | p=0.1274 | p=0.9998 | - |
*p<0.05
At day 7, a highly significant difference was observed in the optical density values of three Groups (I, II, III) (F=8.7062, p=0.0005) at 5% level of significance. It implies that, the antibacterial activity at the end of day 7 was different for three groups [Table/Fig-13]. Further, pairwise comparisons were done by using Tukey’s multiple post hoc test. It was observed that the antibacterial efficacy of Group II (Mean OD=0.2315) was better than group I (Mean OD=0.3884) and Group III (Mean OD=0.3271) on 7th day. Also, Group II at the end of day 7 and Group I at the end of day 2 (p=0.0023), Group II at the end of day 7 and Group I at the end of day 7 (p=0.0010) and Group II at the end of day 7 and Group III at the end of day 2 (p=0.0500) show statistically significant difference in the optical density values at 5% level of significance. It implies that, the optical density is significantly lower in Group II at the end of day 7 as compared to Group I at the end of day 2 and day 7 and Group III at the end of day 2 [Table/Fig-12]. This reveals that Ca(OH)2 with imipenem was most effective against E. faecalis to a depth of 400 μm on 7th day.
Comparison of optical density values of three Groups (I, II, III) at the end of day 7 by One way ANOVA.
| Source of variation | Sum of squares | Degrees of freedom | Mean sum of squares | F-value | p-value |
|---|
| Between groups | 0.25 | 2 | 0.13 | 8.7062 | 0.0005* |
| Within groups | 0.82 | 57 | 0.01 |
| Total | 1.07 | 59 | |
*p<0.05
Discussion
Amongst microorganisms, E. faecalis is most commonly implicated in asymptomatic persistent infections [7]. It has been demonstrated that E. faecalis is highly resistant to Ca(OH)2. A combination of two medicaments may produce additive or synergistic effects. Therefore, different materials have been added to Ca(OH)2 in an attempt to enhance its antimicrobial activity against E. faecalis [20-22].
Octenidine is an antiseptic and it is proposed as an alternative to Chlorhexidine (CHX) and Ca(OH)2. Studies have shown that Octenidine was found to be more effective than 5.25% NaOCl against E. faecalis after different intervals in broth dilution test [23] and more effective than 2% CHX against E. faecalis both at 200 μm and 400 μm [24,25]. Hence, it was chosen as one of the test materials in this study.
Imipenem is a beta-lactam carbapenem and has an extremely broad spectrum of activity. This is used in the medical field for the treatment of E. faecalis infection in severe acute pancreatitis and urinary tract infections. Because of its efficacy against E. faecalis, imipenem was chosen as another test drug in this study [26].
The model suggested by Haapasalo M and Orstavik D [18] was altered for the present study. In this study, extracted human teeth were used instead of the bovine incisors [16]. This adjustment was done because of the pronounced variation in the canal diameter of bovine and human teeth. Moreover, it is evident that studies with human dentin blocks would be more appropriate to simulate the clinical situation [17]. Also, cementum was expelled from the samples because the presence of the cementum influenced the capacity of E. faecalis to contaminate the dentinal tubules. In the present study, before the application of the medicament, two coats of nail varnish were applied on the outer surface of the blocks to close the tubule openings thereby, preventing diffusion of intracanal medicament [18].
Many previous studies undertaken using Haapasalo M and Orstavik D model [18] had incubated dentinal blocks for three weeks [27,28]. This is so because three weeks incubation period was sufficient for the formation of clumps of bacteria bounded by a carbohydrate matrix, which was a feature normally found in ‘biofilm’ [29-32]. Hence, in this study bacterial incubation was done for 21 days.
Calcium hydroxide requires a minimum of seven days as an intracanal dressing. Also, the effective antimicrobial action of the tested intracanal medicaments decreases after 48 hours [33]. Hence, this explains the reason why antibacterial activity of calcium hydroxide and other experimental medicaments was evaluated at the end of day 2 and day 7 [34].
The methodology used in this study for evaluation of the antibacterial activity was a modification of the one previously described by Haapasalo M and Orstavik D where they used counts of colony forming units [16,18]. However, in this study optical density of the broth, proportional to the number of viable bacteria present in the dentin debris was measured using the ELISA-reader which is a more accurate method [35,36].
In the present study, it was seen that Group I exhibited the least antibacterial efficacy against E. faecalis at the end of day 2 and day 7. The reason for this limited antibacterial effect of calcium hydroxide on E. faecalis could be attributed to the dentin buffering effect. In order to have antibacterial effect within dentinal tubules, the ionic diffusion of Ca(OH)2 should surpass the dentin buffer capacity, reaching pH levels sufficient enough to destroy bacterial strains particularly E. faecalis which can survive a high pH of 11.5 [37]. Another factor is the arrangement of the E. faecalis colonizing the root canal walls. This can reduce the antibacterial effects of calcium hydroxide, since the cells situated at the periphery of colonies can shield those present at a much deeper level inside the tubules [27]. Furthermore, as E. faecalis forms biofilm on the root dentin, they exhibit the ability to calcify, which may facilitate its stability [37].
It was seen that there was difference in the optical density among the three experimental groups on day 2. This indicates that all the three groups exhibited antibacterial activity on the 2nd day however, Group II exhibited highest antibacterial activity compared to Group I and Group III. This can be attributed to the wide spectrum of antibacterial activity of imipenem and its strong susceptibility to E. faecalis even at very small concentrations.
In addition, the antibacterial efficacy of Group III was seen to be better than Group I on day 2. This could be attributed to broad spectrum activity and a different class of chemicals of octenidine that is bispyridinamines which displays unique properties not common to other cationic antiseptic agents [38,39]. This was in accordance with the results obtained by other authors like De Lucena JM et al., [12]. To add, Haapasalo M et al., [18] in their clinical trials found that Octenidine was able to achieve similar results like CHX against E. faecalis. Tandjung L et al., and Sundqvist G et al., in their respective studies also found that octenidine acted as a potent antimicrobial agent capable of reducing the amount of E. faecalis found in infected areas of endodontic therapy [13,40].
The slight decrease in the antibacterial efficacy of calcium hydroxide in Group I from 2nd to 7th day could be due to the vehicle used in this group which was saline in this case. Saline being a water based vehicle diffuses rapidly leading to decreased availability of hydroxyl ions responsible for antibacterial activity of calcium hydroxide, thereby by lowering its antibacterial activity by 7th day. These results were in accordance with those obtained by Vaghela DJ et al., [17]. The increased antibacterial activity of Group II and Group III on day 7 inspite of the same vehicle used could be attributed to the synergistic effect. This leads to enhanced activity that occurs when two medicaments are combined together. Similar results were seen by Tirali RE et al., in their study where octenidine was mixed with calcium hydroxide [41]. Also, De Lucena JM et al., found that when octenidine gel was placed for seven days in root canal system, no bacterial growth was observed [12].
Antibacterial activity of Group II on 7th day was significantly better than Group I and Group III. This revealed that calcium hydroxide with imipenem for 7 days proved to be the most effective medicament against E. faecalis. This is so because imipenem acts as an effective antimicrobial agent by inhibiting cell wall synthesis of various Gram-negative and Gram-positive bacteria. It remains very stable even in the presence of β-lactamase (both cephalosporinase and penicillinase) produced by some bacteria including E. faecalis and is a strong inhibitor of β-lactamases from some Gram-negative bacteria that offer resistance to most β-lactam antibiotics. Thus, this plays an important role in the treatment of infections which are not readily treated with other antibiotics [42].
These results were in accordance with those obtained by Gaetti-Jardim EC et al., and Gaetti-Jardim Júnior E et al., who concluded that Imipenem is effective against the microbial flora isolated from endodontic infections. [14,15].
Limitation
One limitation of this study is that a single organism was used to infect the root canal. Because endodontic infections are polymicrobial, interactions between multiple organisms could potentially have different dynamics than were demonstrated by this study. The intracanal medicament that is effective against single microbe in vitro may not necessarily be effective against the same microbe in vivo since the root canal system contains multiple microorganisms.
Also in this study, the maximum duration for which medicament was left in the canal was for seven days. Further research is warranted where medicament should be left for a longer duration to better understand their antibacterial action against E. faecalis. However, care should be taken with the use of antibiotics to avoid development of resistance.
Conclusion
Thus, under the conditions of the present study, it was concluded that all the tested medicaments in this study exhibited antibacterial activity against E. faecalis on day two and day seven but the most potent medicament against E. faecalis is the combination of imipenem with Calcium hydroxide for seven days. Combination of octenidine hydrochloride with calcium hydroxide offered better antibacterial efficacy than calcium hydroxide with sterile saline but less than the combination of imipenem with calcium hydroxide at both the time intervals i.e. day two and day seven.
*p<0.05
*p<0.05