The role of FnECHO for the evaluation of haemodynamic status in stable as well as sick infants has been well established over the last few years [1,2]. Early recognition of cardiovascular compromise in sick infants enables the physician to take timely therapeutic decisions and monitor response to treatment more objectively [3,4].
Neonatal sepsis is a cause of significant short term as well as long term morbidity and mortality among term and preterm infants [5,6]. Circulatory abnormalities are variable and may include vasodilatation with decrease in systemic vascular resistance, intravascular volume depletion, vasoconstriction or myocardial depression [7,8].
The haemodynamic status of sick newborn infants is often assessed by clinical variables such as heart rate, blood pressure and capillary refill time which have been demonstrated to be misleading in their accuracy [9,10]. It has been demonstrated in several studies that real time objective physiologic data such as cardiac output and other measurements of central blood flow provide haemodynamic information, which may be different from the assumed underlying physiology [4,11]. The most commonly used measurements for evaluation of central blood flow in newborn infants are RVO, LVO and Superior Vena Cava (SVC) flow [12,13].
However, there is a paucity of data on haemodynamics in infants with sepsis. Furthermore, data specific to Indian settings is sparse. As per a recently conducted large multicentre study on neonatal sepsis in India, two-thirds of the isolates in culture positive sepsis were gram-negative pathogens [5]. There are very few studies which have specifically studied the haemodynamic changes in gram-negative neonatal sepsis or attempted to compare the findings with those in gram-positive sepsis. Neonatal sepsis is classified as early onset and late onset. Late onset sepsis is defined as onset of sepsis after 72 hours of life [14]. In this study, we aimed to understand better, the changes in central blood flow in neonates diagnosed with late onset sepsis, by evaluating the RVO and LVO with the help of FnECHO.
Materials and Methods
This prospective cohort study was conducted at the tertiary level neonatal unit of a university medical college of Western India from March 2015 to November 2015.
All infants admitted in the NICU who had suspected late onset sepsis on the basis of clinical and laboratory findings were enrolled in the study. Majority of neonates in our unit with culture positive, proven sepsis have late onset sepsis. Many patients with early onset sepsis were outborn and were admitted to the unit after receiving antibiotics and/or inotropic support, which often affects the culture reports as well as haemodynamics. Only neonates with late onset sepsis were therefore included in the study. Based on a sepsis score created by Töllner U [15], and also from observations of the most common clinical presentations of sepsis in our unit, we defined suspected sepsis if the neonate had three or more of the following categories of clinical signs: Haemodynamic instability- delayed capillary refill time, hypotension, unexplained tachycardia; Metabolic derangement- metabolic acidosis, hypoglycaemia or hyperglycaemia; Respiratory distress- tachypnoea, grunting, chest retractions; Unexplained apnoeic spells; Temperature instability- hypothermia or fever; Feed intolerance- vomiting, abdominal distension; Neurological symptoms-lethargy, seizures, muscular hypotonia.
With every suspected infection, a sepsis screen was performed which included a full blood count with immature to total neutrophil (IT) ratio, C-Reactive Protein (CRP), blood culture and lumbar puncture. Urine culture and chest or abdominal radiography was performed if indicated. All infants with suspected sepsis underwent FnECHO within 12 hours of onset of clinical signs, before starting inotropic support if needed. Demographic details, clinical examination findings and measurements of RVO and LVO were recorded. Infants with positive culture results (proven late onset sepsis) were included in the final analysis.
Infants with early onset or culture negative sepsis, perinatal asphyxia, congenital heart disease, major congenital malformations and genetic syndromes were excluded from the study.
Approval from institutional Ethics Committee was obtained and informed consent was taken from parents of the infants eligible for the study.
The FnECHO was performed by a neonatologist (PS) with core training and experience of more than five years in FnECHO, using a Siemens ultrasound machine (Acuson X 300, Siemens Medical Solutions, Inc. USA) with neonatal probe (4-8 MHz transducer). Infection control measures were taken while performing the scans. Cardiac blood flows -RVO and LVO were estimated as per previously published methodology described by Evans N and Kluckow M [16].
RVO measurement: The parasternal long axis view was used to record flow at a level just distal to the pulmonary valve by Pulsed Doppler. The maximum velocity time integral was calculated by averaging the values from area under the curve, for the five consecutive cardiac cycles. The peak to peak intervals of the Doppler velocity time signals were used to calculate the heart rate. By using a frame to frame analysis of the grey-scale parasternal long-axis image, the diameter of the pulmonary valve insertion was measured at end systole. The average diameter was calculated from values of the five cardiac cycles.
LVO measurement: The apical view was used to image the left ventricular outflow tract so that the full length of the ascending aorta could be incorporated. The range gate of pulsed Doppler was placed distal to the aortic valve and the maximum velocity time integral was calculated by averaging the flow velocity time signal values of five consecutive cardiac cycles. The peak to peak intervals of the Doppler velocity time signals were used to calculate the heart rate. A parasternal long axis view was used to measure the internal diameter of the ascending aorta at the site of flow analysis, at the end of systole, from a frame by frame analysis of the Gray-scale image. An average value of the diameter was calculated from five cardiac cycles.
The following formula was used for measuring ventricular output:
LVO/RVO= HRx VTI x π (d/2)2Where, Heart Rate (HR), Velocity Time Integral (VTI)-aortic or pulmonary artery VTI, d-diameter of the outflow tract, π -3.142.
Ventricular output was represented as mL/kg/minute. Normal range of LVO and RVO was defined as 150 to 300 mL/kg/minute each [17-19]. Values above and below the limits of normal range were categorised as high and low cardiac output respectively.
Analysis of Data
Qualitative data were presented as frequencies and percentages, whereas quantitative data were presented as mean, standard deviation, or median and range. For comparison of means of two independent groups, unpaired t-test was used. The data was analysed using SPSS version 20.0. A p-value of <0.05 was considered as statistically significant.
Results
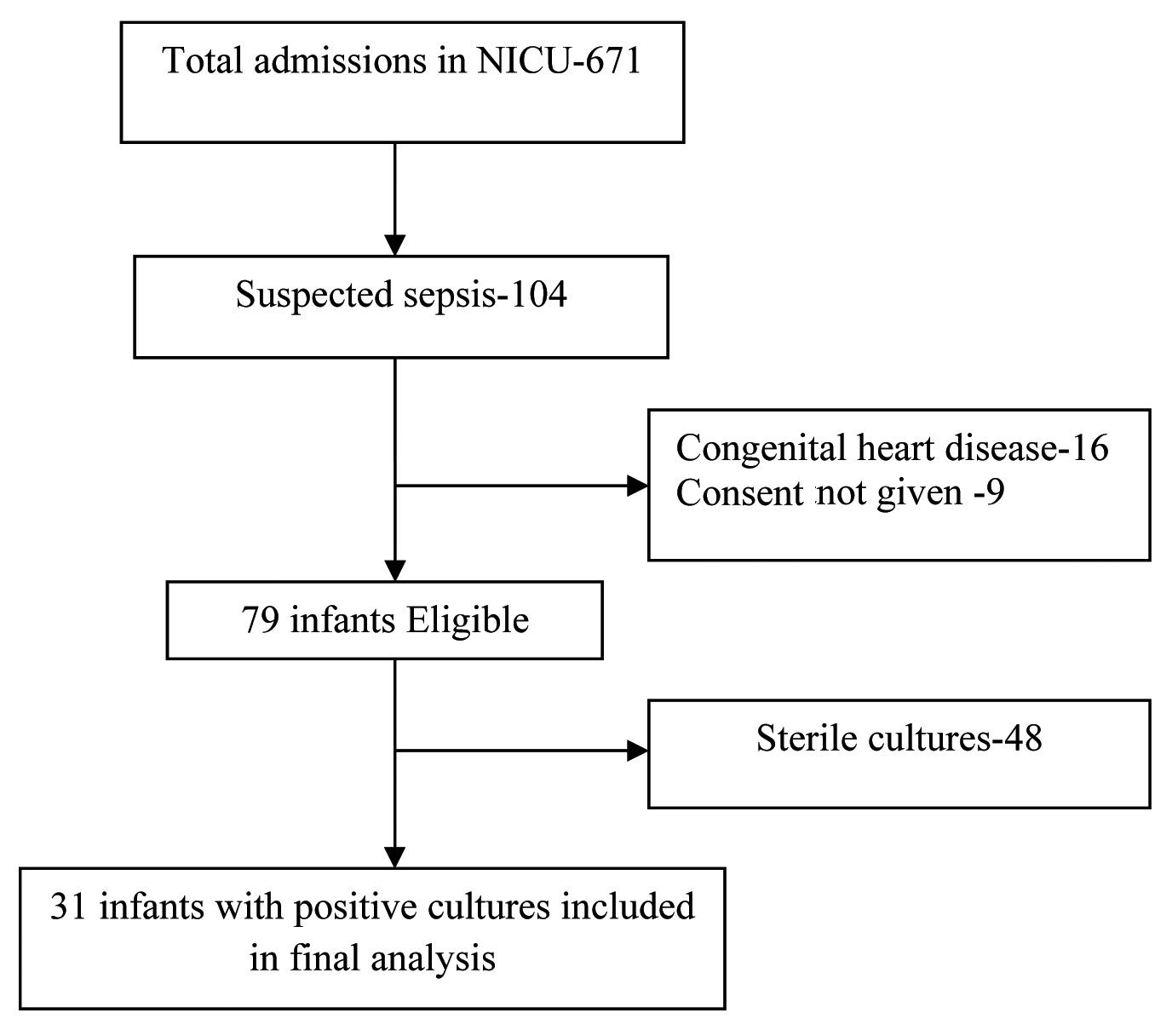
A total of 671 infants were admitted in the NICU between March 2015 and November 2015. Of these, 104 infants were identified to have suspected sepsis. Thirty one infants with late onset culture- positive sepsis met the inclusion criteria and were eligible for final data analysis [Table/Fig-1].
Flow diagram of the study cohort.
Demographics of study patients (n=31)
| Characteristic | Values |
|---|
| Median (range) admission weight (gm) | 1430 (578-3120) |
| Preterm n (%) | 21 (67.7%) |
| Very preterm (<32 weeks) n (%) | 15(48.4%) |
| Low birth weight n (%) | 26 (83.8%) |
| SGA n (%) | 19 (61.3%) |
| Male/Female n (%) | 17 (54.8%)/14(45.2%) |
| Median (range) postnatal age at onset of sepsis (d) | 6 (4-34) |
n-number, SGA-small for gestational age, d- days
[Table/Fig-2] summarises the demographic characteristics of the included infants. Median admission weight was 1430 gm. Twenty one neonates (67.7%) were preterm and 15 (48.4%) were very preterm (<32 weeks). The median (range) postnatal age of onset of sepsis was six days (4-34).
Distribution of Organisms Isolated in Blood Culture
It was observed that 24 patients (77.4%) in the study had gram-negative sepsis. Klebsiella pneumoniae was the most common organism isolated (15, 48.4%) [Table/Fig-3].
Distribution of organisms isolated in blood culture (n=31).
| Organism isolated | Number of patients (percentages) |
|---|
| Klebsiella pneumoniae n (%) | 15 (48.4%) |
| Escherichia coli n (%) | 5 (16.1%) |
| Acinetobacter n (%) | 3 (9.7%) |
| Proteus mirabilis n (%) | 1 (3.2%) |
| Staphylococcus aureus n (%) | 5 (16.1%) |
| Coagulase negative Staphylococcus (CONS) n (%) | 2 (6.4%) |
Ventricular Output in Gram Negative and Gram Positive Sepsis
Mean (±SD) RVO and LVO of the infants with late onset sepsis in our study group were 313 mL/kg/minute (±110.4) and 347 mL/kg/minute (±139.9) respectively, which were higher than normal values. When further analysed according to the nature of the infecting organism [Table/Fig-4], it was noted that mean RVO and LVO were significantly higher in patients with gram-negative sepsis (338 and 378 mL/kg/minute respectively), while remaining in the normal range in patients with gram-positive sepsis (225 and 240 mL/kg/minute respectively).
Comparison of ventricular outputs in gram-negative and gram-positive sepsis
| OrganismIsolated | Number of patients | Mean (±SD) ventricular output | p-value* |
|---|
| RVO | Gram-Negative | 24 | 338.0 (±107.7) | 0.014 |
| Gram-Positive | 7 | 225.0 (±69.9) |
| LVO | Gram-Negative | 24 | 378.0 (±138.4) | 0.02 |
| Gram-Positive | 7 | 240.0 (±86.1) |
*Unpaired t-test was used
SD- Standard deviation, RVO-right ventricular output, LVO- left ventricular output
Ventricular Output In Term and Preterm Infants
There was no significant difference in the mean ventricular outputs in term and preterm infants with late onset sepsis [Table/Fig-5].
Right ventricular and left ventricular output in term and preterm infants with late onset sepsis
| Term (n=10)Mean (±SD) | Pre term (n=21)Mean (±SD) | p-value† |
|---|
| RVO | 338.2 (±122.95) | 301.0 (±105.01) | 0.422 |
| LVO | 376.6 (±136.61) | 333.0 (±142.61) | 0.424 |
† Unpaired t-test was used
RVO-right ventricular output, LVO-left ventricular output
Discussion
In our study, we observed that two-thirds of infants in NICU who had late onset culture positive sepsis were preterm, with a large majority being very preterm (<32 weeks gestation). Majority of the infants with culture proven late onset sepsis had gram-negative sepsis, with Klebsiella pneumoniae being the most common organism isolated. This was consistent with the data reported from the other neonatal centres of India [20-22], as well as the large multicentre Delhi Neonatal Infection Study [5].
The mean RVO and LVO values were higher than normal in infants with late onset sepsis. The higher values of cardiac outputs were seen in patients with gram-negative sepsis, while remaining in the normal range with gram-positive sepsis. This indicates that a pattern of low systemic vascular resistance with increased cardiac output is a predominant feature of gram-negative sepsis. These findings of higher cardiac outputs in patients with gram-negative sepsis were seen in term as well as preterm infants. Saini S et al., evaluated haemodynamic changes in 52 preterm infants with septic shock [23], using FnECHO and found an elevated LVO in preterm infants with septic shock. Majority of infants in their study had gram-negative infection, with Klebsiella pneumoniae being the most common organism isolated.
Findings of our study showed an increased RVO and LVO were consistent with those of similar studies, most of which included preterm infants with sepsis [24,25]. Murase M et al., performed echocardiographic assessments consecutively from birth to day 28 in 13 Very Low Birth Weight (VLBW) infants, who were diagnosed with prenatal infections at birth. The mean LVO and stroke volume were both significantly higher in the infectious group than in the control group at 12 and 72 hours of life [24]. In an observational cohort study, de Waal K and Evans N [25] measured blood pressure, RVO, LVO, and SVC flow of twenty preterm infants who had a suspected infection and signs of cardiovascular compromise. The infants with sepsis had relatively high left and right cardiac outputs and low Systemic Vascular Resistance (SVR). Brierley J and Peters M prospectively studied 30 children with suspected fluid-resistant septic shock admitted to the paediatric intensive care unit. It was observed that children with central venous catheter-associated infection presented with “warm shock”, which was characterised by high cardiac index and low SVR. In contrast, a normal or low cardiac index was predominant in patients with community-acquired sepsis [26].
The difference in pathophysiology and host response in gram-negative and gram-positive bacteraemia could explain the differences in haemodynamics. It has been proven in animal studies that the nature of endotoxins produced by gram-negative bacteria (for e.g., the lipid A moiety of lipopolysaccharide complex present on bacterial cell wall) is responsible for the haemodynamic alterations characterised by marked hypotension and decrease in systemic vascular resistance [27]. Suffredini A et al., evaluated the haemodynamic effects of endotoxins (Escherichia coli) in humans by administering intravenous purified lipopolysaccharide to normal subjects [28]. Three hours after the administration of endotoxin and before volume loading, systemic vascular resistance and mean arterial pressure had decreased by 46 and 18%, respectively, while the cardiac index had increased by 53%.
Haemodynamic assessment in sepsis needs to be frequent as it is a dynamic situation and the findings change with the course of the condition [29]. It has been demonstrated in several studies that an objective assessment of haemodynamic status by FnECHO helps the attending physician to customise choice of cardiovascular support and monitor the response to treatment [4,30].
Although, there are studies which have measured RVO and LVO for haemodynamic assessment of infants with sepsis, Indian data on this subject in the neonatal population is scarce. As far as we are aware, our study is the first prospective cohort study comparing the cardiac outputs of infants with gram-negative sepsis and those with gram-positive sepsis.
Limitation
First, the study population was small. Second, as haemodynamics in sepsis changes with time, serial measurements of haemodynamic variables could have provided information on clinical progress and response to therapy, as well as predictors of mortality if any. However, this was not accomplished in the present study. Future research could be done to tailor treatment as per the echocardiographic findings in a randomised manner and analyse outcomes, as this was not evaluated in the current study.
Conclusion
Neonatal sepsis is a unique haemodynamic state and neonates with late onset sepsis show high right and left ventricular outputs as demonstrated by FnECHO. These higher cardiac output values are predominantly seen in patients with gram-negative sepsis as compared to those with gram-positive sepsis. Further larger studies are needed to evaluate whether treatment decisions could be taken, based on measurements of cardiac outputs by FnECHO, in neonates with suspected sepsis.