Interstitial Lung Diseases (ILDs) are a diverse group of pulmonary disorders classified together because of similar clinical, roentgenographic, physiologic, or pathologic features. Pathologically, the interstitial lung diseases are characterized by a varying degree of fibrosis and inflammation of the lung parenchyma or interstitium [1].
HRCT is widely recognized as a sensitive and specific modality for the assessment of diffuse lung processes, most notably the idiopathic interstitial pneumonias, eosinophilic lung diseases, and obstructive lung diseases [2].
HRCT though a sensitive modality, suffers from limitations of radiation exposure, making it unsuitable for use in pregnancy and children. Moreover, it is not available at primary and secondary levels of health care. Spirometry has the advantage of being free from the hazards of radiation exposure. It is a low-cost alternative to HRCT and does not require an experienced radiologist for its interpretation. Its importance also lies in the fact that it provides a fair assessment of pulmonary function, an aspect which cannot be evaluated by HRCT [1,2].
The aim of this study hence, was to establish a correlation between HRCT and spirometry, so as to ascertain if spirometric indices can be used as an economical alternative to establish the severity of disease.
Materials and Methods
The study was hospital-based cross-sectional study conducted during November 2014 to August 2016. The Institutional Review Board approval for conducting this study was obtained and informed consent for study was taken from all patients. A total of 60 patients referred to Department of Radio-Diagnosis, Rajindra Hospital, Patiala with clinical diagnosis of ILD were included in this study. Both male and female patients with clinical profile of dyspnea and chronic cough and X-ray findings suspicious of ILD were included in this study. Patients of varied socioeconomic strata and literacy levels were included. Cases of infective aetiology (HIV, Tuberculosis etc.,) or malignant aetiology as well as chronic obstructive pulmonary disease were excluded. Detailed proforma was filled for all those patients who met with the inclusion criteria. The proforma included the patient’s name, age, sex, address, registration number, complaints, risk factors, previous medical or surgical history, laboratory investigations and spirometric findings. Complaints were noted in detail and the severity grading of various complaints were done e.g., dyspnoea, measured as per modified Medical Research Council (mMRC) Dyspnoea scale [3], Dry Cough (based on 10-point Visual Analogue-type response scales (VAS) [4], Generalized weakness (based on patient’s subjective perception), Weight loss (Unintentional weight loss, of at least 5% of the patient’s usual body weight within the preceding 6 to 12 months), Chest pain (VAS, scale 1-10) [5-7] and Joint pain (based on patient’s subjective perception) etc., Using the standard protocol, HRCT chest was done on 6 slice Siemens-Somatom CT scanner in supine position and it was read by a single experienced radiologist. Parenchymal abnormalities were categorized into four main and 12 other associated features with their distribution along the lung zones. HRCT severity score [Table/Fig-1] was calculated based on Semi-quantitative scoring method used by Warrick JH et al., [8].
Correlation between severity and extent scores.
| Severity score | Extent score |
|---|
| Abnormality | Grading | Bronchopulmonary segments – for each abnormality, score by number of segments involved | Grading |
| Ground-glass opacitiesIrregular pleural marginSeptal or subpleural linesHoneycombingSubpleural cyst | 12345 | 1 to 3 segments involved4 to 9 segments involved>9 segments involved | 123 |
| Maximal severity score | 15 | Maximal extent score | 15 |
Spirometry was performed using Medisoft Spiro Air dry rolling seal spirometer. The details of the procedure were explained to all patients and a written consent was obtained from all. Patients exhaled for at least six seconds and stopped when there was no volume change for one second. At least three acceptable spirograms were obtained with a maximum of 8 trials. The largest FVC and FEV1 from acceptable curves were obtained according to American Thoracic society guidelines. Thereafter, these spirometry indices were correlated with HRCT scores.
Statistical Analysis
Microsoft Excel version 2007 was used for Standard statistical analysis. Categorical variables were presented in number and percentage (%) and continuous variables were presented as mean ± SD and median. Age was compared using Unpaired t-test between male and female. Qualitative variables were correlated using Chi-Square test/Fisher’s-exact test. Pearson correlation coefficient was used to correlate scores with FEV1, VC and FVC. A p-value of <0.05 was considered statistically significant. The data was entered in MS EXCEL spreadsheet and analysis was done using Statistical Package for Social Sciences (SPSS) version 21.0.
Results
Majority of the patients 46.67% (n=28) were between the ages of 51-70 years, and comprised of 13 males and 15 females. The major complaints were gradual onset dyspnea/dyspnea on exertion, followed by dry cough and generalized weakness. Few patients had associated symptoms like fever, joint pain, chest pain and weight loss etc.
The most common interstitial lung disease found in our study was Usual Interstitial Pneumonia (UIP)/Idiopathic Pulmonary Fibrosis (IPF) (n=33; 55%) followed by nonspecific interstitial pneumonia (NSIP) (n=9; 15%), sarcoidosis (n=5; 8.3%), hypersensitivity pneumonitis (HSP, n=5; 8.3%), respiratory bronchiolitis associated RB-ILD (n=1; 1.67%), Desquamative Interstitial Pneumonia (DIP, n=1;1.67%) and unclassified Idiopathic Interstitial Pneumonia (IIP, n=6; 10.0%).
The most common finding observed on HRCT was septal or subpleural lines. Ground Glass Opacities (GGO’s), irregular pleural margins and honeycombing were also commonly observed [Table/Fig-2].
Common HRCT patterns associated with ILD and their distribution in the present study.
| HRCT Features in ILD patients (n=60) | No of Patients | Percentage |
|---|
| Septal/subpleural lines | 52 | 86.67% |
| Ground Glass Opacities (GGO’S) | 45 | 75.00% |
| Irregular pleural margins | 44 | 73.33% |
| Honeycombing | 33 | 55.00% |
| Microcystic honeycombing | 6 | 10.00% |
| Tractional bronchiectasis | 32 | 53.33% |
| Nodules | 30 | 50.00% |
| Tractional bronchioloectasis | 14 | 23.33% |
| Consolidation | 7 | 11.67% |
| Subpleural cysts | 6 | 10.00% |
| Mosaic attenuation | 14 | 23.33% |
| Bronchovascular thickening | 12 | 20.00% |
| Emphysematous changes | 5 | 8.33% |
| Mediastinal lymphadenopathy | 9 | 15.00% |
| Architectural distortion | 17 | 28.33% |
| Pleural effusion | 2 | 3.33% |
| Distribution |
| Middle/lingual | 41 | 68.33% |
| Lower | 48 | 80.00% |
| Upper | 16 | 26.67% |
| Subpleural sparing | 17 | 28.33% |
Severity score of all the ILD patients was calculated, using a semi-quantitative scoring system based on study by Warrick JH et al., [8], wherein GGO’s, Septal/subpleural lines, pleural irregularities, honeycombing and subpleural cysts were taken into consideration. Extent scores were based on number of segments involved. [Table/Fig-3,4].
Severity score in various types of ILD.
| Provisional Diagnosis | HRCT severity score 0-5 | 6-10 | 11-15 | Total |
|---|
| UIP | 4 (22.22%) | 22 (66.67%) | 7 (77.78%) | 33 (55.00%) |
| NSIP | 2 (11.11%) | 7 (21.21%) | 0 (0.00%) | 9 (15.00%) |
| SARCOIDOSIS | 4 (22.22%) | 1 (3.03%) | 0 (0.00%) | 5 (8.33%) |
| HSP | 4 (22.22%) | 1 (3.03%) | 0 (0.00%) | 5 (8.33%) |
| RB-ILD | 0 (0.00%) | 0 (0.00%) | 1 (11.11%) | 1 (1.67%) |
| DIP | 0 (0.00%) | 0 (0.00%) | 1 (11.11%) | 1 (1.67%) |
| UNCLASSIFIED IIP | 4 (22.22%) | 2 (6.06%) | 0 (0.00%) | 6 (10.00%) |
| Total | 18 (100.00%) | 33 (100.00%) | 9 (100.00%) | 60 (100.00%) |
Extent score in various types of ILD.
| Provisional Diagnosis | HRCT Extent score 0-5 | 6-10 | 11-15 | Total |
|---|
| UIP | 3 (25.00%) | 7 (46.67%) | 23 (69.70%) | 33 (55.00%) |
| NSIP | 2 (16.67%) | 0 (0.00%) | 7 (21.21%) | 9 (15.00%) |
| SARCOIDOSIS | 2 (16.67%) | 2 (13.33%) | 1 (3.03%) | 5 (8.33%) |
| HSP | 1 (8.33%) | 4 (26.67%) | 0 (0.00%) | 5 (8.33%) |
| RB-ILD | 0 (0.00%) | 0 (0.00%) | 1 (3.03%) | 1 (1.67%) |
| DIP | 0 (0.00%) | 0 (0.00%) | 1 (3.03%) | 1 (1.67%) |
| UNCLASSIFIED IIP | 4 (33.33%) | 2 (13.33%) | 0 (0.00%) | 6 (10.00%) |
| Total | 12 (100.00%) | 15 (100.00%) | 33 (100.00%) | 60 (100.00%) |
Spirometry was performed in all the cases. Majority of the patients, 36.67% (n=22) in the study showed severely low FVC. 16.67% (n=8) had moderately low and 23.33% (n=14) had mildly low FVC. 23.33% of patients had severe affection of VC (n=14). FEV1 of majority of the patients were normal i.e., 33.33%, (n=20) of ILD, showed a normal FEV1 where as 21.67% had a moderate affection of FEV1 and only 1.67% had very severe affection of the FEV1.
The correlation between the HRCT severity and spirometric severity is established as in [Table/Fig-5]. The HRCT images showing changing indicating IPF, NSIP, HSP, sarcoidosis, DIP and unclassified IIP [Table/Fig-6,7,8,9,10 and 11].
Correlation between HRCT scores.
| Variables | HRCT SeverityScore | HRCT extentScore |
|---|
| Forced Vital capacity (FVC%) | Correlation Coefficient | -0.578 | -0.506 |
| Significance Level P | <0.0001 | <0.0001 |
| N | 60 | 60 |
| Vital Capacity (VC%) | Correlation Coefficient | -0.295 | -0.259 |
| Significance Level P | <0.0001 | <0.0001 |
| N | 60 | 60 |
| Forced Expiratory Volume in 1st second (FEV1%) | Correlation Coefficient | -0.271 | -0.163 |
| Significance Level P | <0.0001 | <0.0001 |
| N | 60 | 60 |
(severity and extent) and PFT (FVC, VC, FEV1)
(Pearson correlation coefficient and SPSS software was used in this table. Qualitative variables were correlated using Chi-Square test/Fisher’s-exact test)
a,b) HRCT shows bilateral diffuse extensive fibrosis with septal thickening, honeycombing (straight arrow), subpleural cysts (open blue arrow) and traction bronchiectasis (curved arrow) having mid and basal predominance in subpleural region bilaterally. Findings indicate usual interstitial pneumonia/idiopathic pulmonary fibrosis.
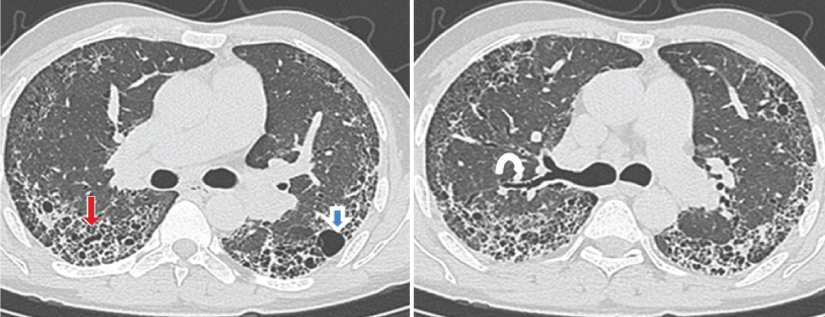
a,b) HRCT images show bilateral diffuse interstitial lung disease in the form of interlobular and intralobular septal thickening (arrowhead) and subpleural lines (arrow). Pleural irregularities are also seen (open blue arrow). Findings indicate Nonspecific Interstitial Pneumonia.
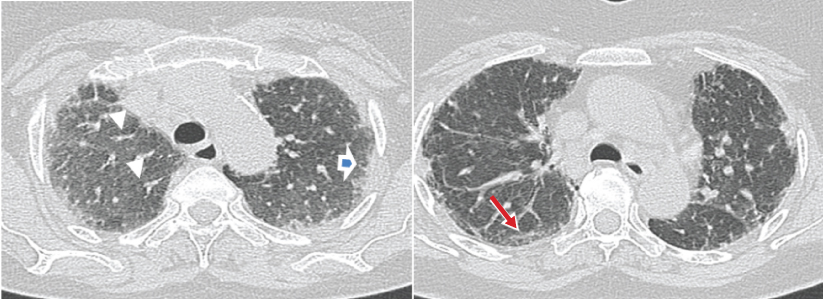
a,b) Show bilateral symmetrical ground glass opacities (white arrows) in mid and basal lung zones with round centri-lobular opacities having geographical distribution of increased, decreased (red arrow) and normal lung attenuation giving head cheese sign. Imaging findings are consistent with Hypersensitivity Pneumonitis.
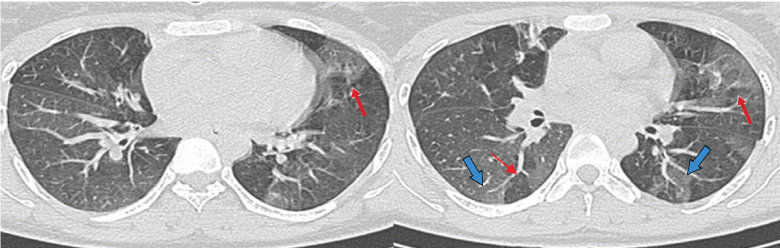
a-c) HRCT images show nodular opacities along fissures and lymphatics. Bilateral hilar (thick red arrows) and right paratracheal (thin white arrow) lymphadenopathy seen giving 1-2-3 sign in a case of Sarcoidosis.
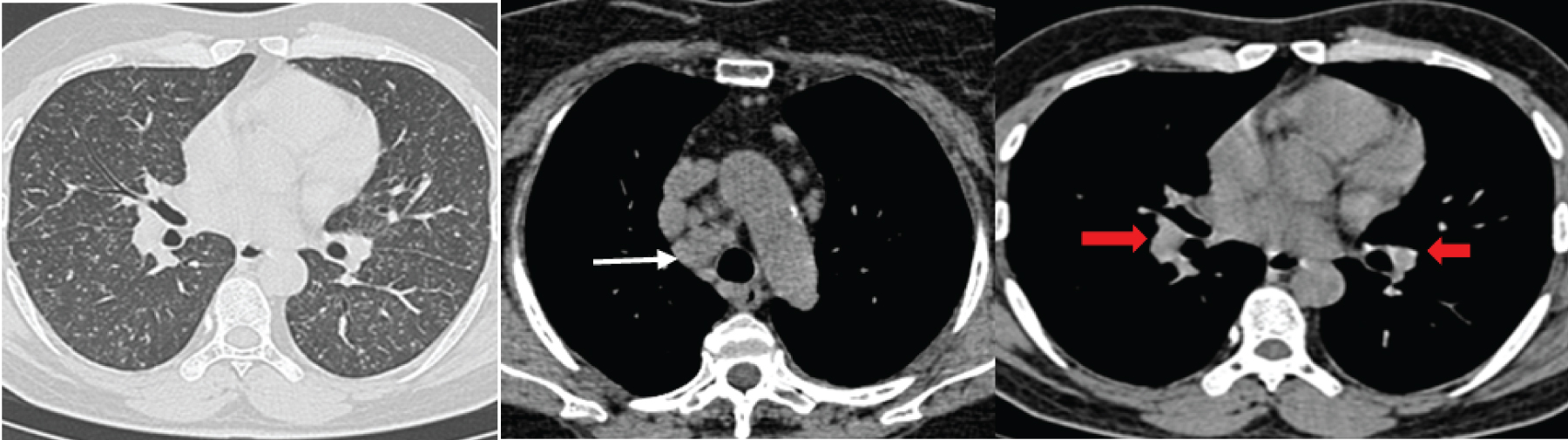
a,b) HRCT images show bilateral symmetrical ground glass opacities with reticular changes more in peripheral region. Bronchioloectatic changes with broncho-vascular interstitial thickening (straight red arrow) and minimal honeycombing (open blue arrow) are seen. Enlarged pulmonary artery and bilateral pleural effusion (star) are also seen. Imaging features are consistent with Desquamative Interstitial Pneumonia.
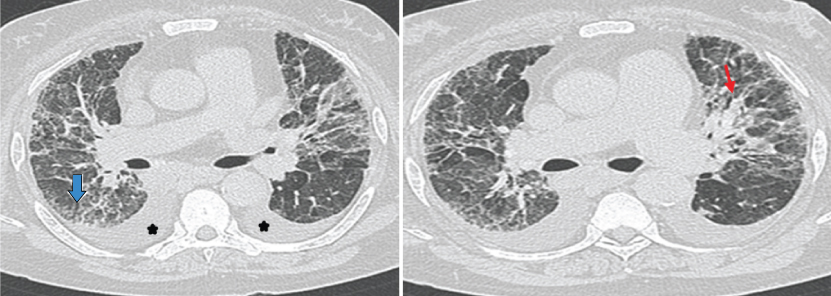
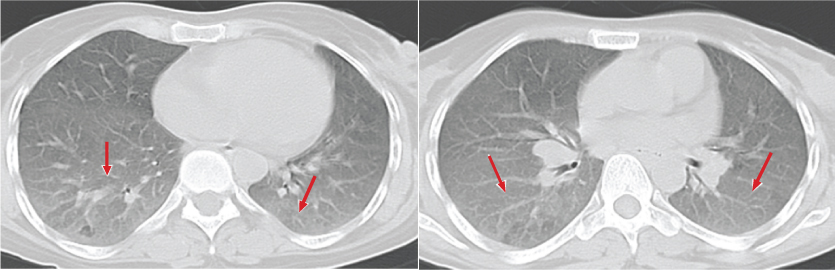
a,b) HRCT image shows areas of ground glass opacities (arrows) and centrilobular nodules superimposed on ground glass opacities. Imaging features and history of smoking are in favour of respiratory bronchiolitis associated interstitial lung disease.
Discussion
Out of sixty cases in our study 54 (90%) cases showed specific patterns of ILD and six cases (10%) showed nonspecific patterns and were classified under unclassified IIP. Analysis of age distribution of patients in our study showed a wide range with maximum percentage of patients 46.67% in the age group 51-70. Study by Jindal SK et al., showed almost similar age of presentation with maximum patients in age group 40-70 years of age [9]. Coultas DB et al., found the age of presentation almost a decade later (61-70 years) [10].
In our study, 56.67% patients were females and 43.33% were males. It closely correlated with the study by Jindal SK et al., in which female and male incidence was found to be 57.4% and 42.4% respectively [9]. Similar results were seen in other studies done by Devi MS and Haorongbam S, and Patil PB et al., [11,12].
In our study, the maximum duration of illness was found to be 5 years and the minimum duration of illness was found to be 2 months. Mean duration of illness of the patients was 3 years with SD of 1.6. Maximum patients presented between 1-5 years. Similar duration of illness was found in Indian study done by Gagiya AK et al., in which, maximum patients presented before 5 years of onset of illness and majority presented between 1-3 years [13].
In our study, the most common presenting complaint was gradual onset dyspnoea (n=57; 95 %), followed by dry cough (n=53; 88.3%) and generalized weakness (n=48; 80%). Some patients also had varying symptoms like fever, joint pain, chest pain and weight loss etc. This was in accordance with study by Patil PB et al., with most common presenting complaint being progressive dyspnea (seen in 96% patients), dry cough (in 74%) and associated joint pain (in 44%) [12]. Study by Perez RL and Weinrib L et al., showed similar clinical profile, with progressive dyspnea on exertion and a dry cough as major complaints [14,15]. According to the study by Bhadke BB et al., predominant symptom of ILD was dyspnea on exertion and the degree of dyspnea was closely related to the disease severity and prognosis [16]. Cough was the second most frequent symptom found in the study.
HRCT in disease spectrum of interstitial lung diseases: In our study the most common interstitial lung disease reported on HRCT was usual interstitial pneumonia (UIP)/Idiopathic Pulmonary Fibrosis (IPF), followed by Nonspecific Interstitial Pneumonia (NSIP), sarcoidosis and hypersensitivity pneumonitis [Table/Fig-12,13].
Comparison of previous done studies with present study in ILDs.
| Study | UIP/IPF | NSIP | Sarcoidosis | HP | RB-ILD | DIP | Unclassified IIP |
|---|
| Sen T et al., [17] | 43% | 18% | 22% | 6% | - | - | - |
| Patil PB et al., [12] | 36% | 14% | 2% | 2% | - | - | - |
| Muhammed SK et al., [18] | 39% | 24% | 13% | 17% | - | - | - |
| Bjoraker JA et al., [19] | 62% | 14% | - | 1% | 2% | 8% | - |
| Flaherty KR et al., [20] | 63% | 19.6% | - | 2.9% | RB-ILD/DIP13% | RB-ILD/DIP13% | - |
| Present study | 55% | 15% | 8.3% | 8.3% | 1.67% | 1.67% | 10% |
UIP/IPF: Usual Interstitial Pneumonia/Idiopathic Pulmonary Fibrosis, NSIP: Non Specific Interstitial Pneumonia, HP: Hypersensitivity Pneumonitis, IIP: Idiopathic Interstitial Pneumonia, RB-ILD: Respiratory Bronchiolitis Interstitial Lung disease, DIP: Desquamative interstitial Pneumonia
HRCT patterns associated with ILDs.
| Study | Study population | Main Findings |
|---|
| Patil PB et al., [12] | 50 patients with clinical suspicion of ILDs | Most commonly found pattern in ILD:Reticular opacity (n=37; 64%), Increased opacity (n=29; 58%), Decreased opacity (n=29; 58%)Most specific HRCT findings: Septal thickening (n=37; 64%), Bronchiectasis (n=26; 52%), Ground glass opacity (n=24; 48%) |
| Leslie KO [21] | | Four basic radiological patterns in ILD(1) increased attenuation/GGO/consolidation, (2) reticulation with parenchymal distortion (fibrosis),(3) nodules (large or small, singular or multiple),(4) mosaic patterns and cysts. |
| Elicker B et al., [22] | | Four general patterns of HRCT in ILD: 1) reticular opacities, 2) nodules, 3) increased lung opacity, 4) decreased lung opacity. Within each of these patterns, other features of the images can help narrow the differential diagnosis. |
| Present study | 60 patients with clinical suspicion of ILD | HRCT patterns seen in present study (n=60): Septal/Subpleural lines: 86.67% (n=52), GGO’S: 75% (n=45), Irregular pleural margins: 73.33% (n=44) Honeycombing: 55% (n=33) patients. Tractional bronchiectasis: 53.33% (n=32), Nodules: 50% (n=30) Tractional bronchioloectasis: 23.33% (n=14), Mosaic attenuation 23.33% (n=14). Bronchovascular thickening: 20% (n=12). Subpleural cysts: 10% (n=6). |
Six cases, did not fit into any particular HRCT imaging pattern of ILD and as the Lung biopsy was not done in all cases, diagnosis could not be reached in these cases. So, these cases are included in unclassified IIP category. [Table/Fig-12,13] shows comparison of previous done studies with present study.
HRCT features of different types of ILDs and various comparison studies are summarized in [Table/Fig-14,15,16,17,18 and 19].
Abnormal HRCT features and its distribution in UIP.
| Study teams | Study population | Main Findings |
|---|
| Palmucci S et al., [23] | | Reticular pattern, with/without traction BronchiectasisHoneycombing appearance, Basal and Subpleural predominance. |
| Bourke SJ [24] | | Honeycombing, Reticular shadowing, Traction bronchiectasis. |
| Wuyts WA et al., [25] | | Peripheral and basal distribution of honeycomb changes with traction bronchiectasis, Irregular interlobular septal thickening, Minimal ground-glass opacity. With presence of all these features, the diagnostic accuracy of CT approaches 90-100%. Honeycombing being the strongest predictor of a diagnosis of UIP. |
| Present study | 60 patients with clinical suspicion of ILD | Predominant HRCT findings in UIP (n=33): Septal/sub pleural lines: 93.94% (n= 31), Tractional Bronchiectasis: 81.82% (n=27), Irregular pleural margins: 78.79% (n=26), Honeycombing: 75.76% (n=25), GGO: 60.61% (n=20)Distribution: Predominantly Lower: 93.94% (n=31) |
Abnormal HRCT features and its distribution in NSIP.
| Study teams | Study population | Main Findings |
|---|
| Palmucci S et al., [23] | | Bilateral ground glass areas, Reticular opacities. |
| Elicker B et al., [22] | | Bibasilar, peripheral, traction bronchiectasis accompanied by ground-glass attenuation: considered diagnostic of NSIP. Reticular abnormalities, with/without traction bronchiectasis, are common. Subpleural sparing and tracking of opacities along lower-zone bronchovascular bundles are findings that correlate with NSIP. Honeycombing is rare in NSIP. |
| Present Study | 60 patients with clinical suspicion of ILD | Predominant findings in NSIP (n=9):Septal/Sub pleural lines: 100% (n=9), Nodules: 100% (n=9), GGO’s: 88.89% (n=8), Irregular pleural margins: 77.78% (n=7), Tractional Bronchioloectasis : 77.78% (n=7), Tractional Bronchiectasis : 44.44% (n=4), Bronchovascular thickening: 77.78% (n=7), Microcystic honeycombing: 55.67% (n=5)Distribution: Predominantly Lower: 88.89% (n=8), Subpleural sparing in 77.78% (n=7). |
Abnormal HRCT features and its distribution in Sarcoidosis.
| Study teams | Study population | Main Findings |
|---|
| Ors F et al., [26] | 45 patients with sarcoidosis. | Most common HRCT findings: Nodule, Micronodule, Ground Glass Opacity (GGO), Consolidation. |
| Elicker B et al., [22] | | Nodules as Hallmark, concentrated around bronchovascularstructures, pleura, and interlobular septa. Hilar adenopathy, an expected finding. Confluence of nodules within larger parenchymal opacities. In late stages: Fibrosis (irregular reticulation, traction bronchiectasis, and confluent masses of fibrotic tissue). |
| Present Study | 60 patients with clinical suspicion of ILD | Predominant findings in Sarcoidosis (n=5): Nodularopacities: 100% (n=5), Septal/subpleural lines : 100% (n=5), Mediastinal LAP: 100% (n=5), GGO’s : 80.00% (n=4)Distribution: Predominantly Upper and Mid: 100% (Both) (n=5). |
Abnormal HRCT features and its distribution in HSP.
| Study teams | Study population | Main Findings |
|---|
| Tateishi T et al., [27] | 112 patients with bird-related HP | Predominant findings in acute and recurrent HP: GGO and centrilobular nodules (decreased with disease progression), Chronic HP and insidious HP: Increased Honeycombing. |
| Hansell DM et al., [28] | 22 patients of Hypersensitivity pneumonitis. | Most common CT pattern: Decreased attenuation and mosaic perfusion (n=19), GGO (n=18), Small nodules (n=12), Reticular pattern (n=8), Areas of decreased attenuation correlated with severity of air trapping. |
| Present Study | 60 patients with clinical suspicion of ILD | Predominant HRCT finding in HSP (n=5): GGO’s: 100.00% (n=5), Nodules: 100.00% (n=5), Mosaic attenuation: 100.00% (n=5), Septal/Subpleural lines: 80.00% (n=4). Distribution: Predominantly Mid and lower: 100% (n=5) (Both), Subpleural sparing in 80.00% (n=4). |
Abnormal HRCT features and its distribution in RB-ILD.
| Study teams | Study population | Main Findings |
|---|
| Park JS et al., [29] | 21 patients of pathologically proven RB-ILD. | Major radiographic findings wereBronchial wall thickening: 76%, Ground-glass opacity: 57%.The predominant initial CT findings:Central bronchial wall thickening: 90%, Peripheral bronchial wall thickening: 86%, Centrilobular nodules: 71%, Ground-glass opacity: 67%. None of these CT findings had a significant zonal predominance. |
| Palmucci S et al., [23] | | Poorly defined centriobular nodule, Centrilobular emphysema, Bronchial wall thickening. |
| Present Study | 60 patients with clinical suspicion of ILD | Predominant HRCT finding in RB-ILD (n=1): GGO’s, Irregular pleural margins, Septal/Subpleurallines Bronchovascular thickening, Honeycombing, Nodules, Tractional Bronchioloectasis, Emphysematous changes, Architectural distortion, Subpleural Sparing seen. |
Abnormal HRCT features and its distribution in DIP.
| Study teams | Study population | Main Findings |
|---|
| Palmucci S et al., [23] | | Diffuse ground glass opacities, Irregular linear opacities, Microcysts |
| Attili et al., [30] | | Bilateral patchy ground glass opacity, Reticular opacities, Subpleural and basal predominance, Rare Honeycombing associated centrilobular emphysema |
| Present Study | 60 patients with clinical suspicion of ILD | Predominant HRCT finding in DIP (n=1), GGO’S, Irregular pleural margins, Septal/subpleural lines, HoneycombingNodules, Tractional bronchioloectasis, Mosaic attenuation,Bronchovascular thickening, Pleural effusionDistribution: In mid and lower, nosubpleural sparing seen. |
Correlation of HRCT severity score and extent score with spirometry: In our study, special efforts were made to establish correlation between HRCT scores and Pulmonary function tests. Correlation between Semi-quantitative HRCT severity score and extent score with the FVC, VC and FEV1 was studied [Table/Fig-3,4,5].
Correlation coefficient between HRCT score and FVC: Present study showed significant inverse correlation of HRCT severity score with the FVC (r= -0.578, p-value <0.0001). These findings correlated well with the similar study done by Xaubet A et al., in 39 untreated patients with idiopathic pulmonary fibrosis [31], in which the HRCT score showed a moderate but significant correlation with FVC (r = -0.46, p = 0. 003). Xaubet A et al., also did the correlational study of HRCT extent with the FVC and observed an inverse relationship (r = -0. 51, p = 0.01) [31]. In our study, also a similar negative correlation between HRCT extent score and Pulmonary function test was found {FVC (r= -0.506) with P-value of <0.0001}. Study done by Isaac BTJ et al., in 2015, in 94 patients of IIP, in South India also showed an inverse correlation between the HRCT scores and FVC (r= −0.48) [32]. Mura M et al., and Best AC et al., had also observed a good negative correlation of FVC with HRCT scores [33,34].
Correlation coefficient between HRCT score and VC: Our present study also showed inverse correlation of HRCT severity score with the VC (r= -0.295, p-value <0.0001). This observation was similar as that seen by Battista G et al., who used HRCT visual score [35]. A decrease in the VC and diffusing capacity of lung for carbon monoxide (DLCO) was observed in serial follow up study of the patients with HRCT progression of the disease. O’Donnell D [36] had opined that in restrictive lung diseases, both the VC and FVC are reduced, but the FVC is usually decreased to a relatively lesser extent, as opposed to our study, in which a greater decrease in FVC was seen as compared to VC.
Correlation coefficient between HRCT score and FEV1: In the present study, negative correlation was also seen between the HRCT severity score and FEV1, with correlation coefficient of (-0.271). This correlation seemed less significant in comparison to the relationship between the HRCT severity with FVC and VC. This was in consistence with the study done by Ooi GC et al., in patients with systemic sclerosis [37], where an inverse correlation between HRCT scores and FEV1 was seen (r=–0.37, p=0.03). Same study found stronger inverse correlation of HRCT score with FVC (r=−0.43, p=0.008).
Biederer J et al., in his study of correlation between HRCT findings and pulmonary function tests, in 53 patients of ILD, observed that lesions in HRCT correlated weakly with FEV1 (r=−0.31; p<0.01) [38]. Same study found a much stronger correlation between the HRCT severity with the diffusion capacity (r=−0.54; p<0.001). As there is a definite correlation between the HRCT severity and spirometric findings, spirometric indices can be used in the follow up of patients with ILD.
Limitation
This was not a prospective study and critically ill patients could not perform spirometric manoeuvers.
Conclusion
Spirometry is a simple, noninvasive modality to measure severity of ILD and help in its objective assessment. It is bereft of all radiation hazards and can be conveniently performed in pregnant patients and young children. Being cheaper it is more readily available than HRCT and it is a powerful tool that can be used to detect and manage patients with restrictive lung disorders. HRCT is invaluable in characterizing the disease process, but it cannot assess the physiological lung functions. As the severity and extent scores of HRCT have an inverse relationship with the spirometric indices in ILD, spirometry can provide a viable alternative to assess the disease severity.
(severity and extent) and PFT (FVC, VC, FEV1)
(Pearson correlation coefficient and SPSS software was used in this table. Qualitative variables were correlated using Chi-Square test/Fisher’s-exact test)
UIP/IPF: Usual Interstitial Pneumonia/Idiopathic Pulmonary Fibrosis, NSIP: Non Specific Interstitial Pneumonia, HP: Hypersensitivity Pneumonitis, IIP: Idiopathic Interstitial Pneumonia, RB-ILD: Respiratory Bronchiolitis Interstitial Lung disease, DIP: Desquamative interstitial Pneumonia