CNS involvement occurs early in HIV patients and is responsible for significant morbidity and mortality. The spectrum of CNS involvement in HIV includes effect of the virus itself, opportunistic infections, certain neoplasms and the effect of treatment. These patients show increased prevalence of certain opportunistic infections with CD4+ counts of less than 200 CD4+ T cells/μL. [1,2].
Cross-sectional imaging plays a key role in patients with CNS involvement in HIV. Computed Tomography (CT) and MRI are the modalities used for imaging the CNS. CT is often the initial modality used, however it has a restricted role due to limited soft tissue resolution. MRI with its multiplanar capability and excellent resolution is the preferred modality of choice for imaging the brain. MRI is utilised for the diagnosis, for follow up and for detecting complications of the disease and the treatment. MRI is also helpful in guiding the appropriate therapy [2].
There has been a dramatic decline in mortality of AIDS patients with the introduction of Highly Active Anti Retroviral therapy (HAART). This study was conducted to analyse the disease pattern amongst HIV patient in Indian population. Our center being one of the referral point for patients of HIV has a high study population and thus the statistical data would bring out the salient features, behavioural pattern of disease and treatment response amongst Indian population. The literature will thus help the clinician in understanding the clinico-radiological pattern in this part of the world. Due to this type of continuous research, AIDS has evolved from a virtual death sentence to a chronic disease [3].
According to National AIDS Control Organization (NACO), India, the estimated number of people living with HIV was 2.08 million in 2011 [4]. The incidence of neurologic manifestations in HIV is as high as 60% in patients with AIDS, as HIV is a neurotropic virus and enters the CNS early in the course of infection [2,5]. HIV crosses the intact blood-brain barrier and can be cultured from the brain parenchyma and cerebrospinal fluid [5,6]. Therefore, this study was carried out to evaluate the spectrum and features of brain parenchymal involvement in HIV patients who presented with neurological symptoms.
Materials and Methods
A retrospective study was conducted at a tertiary care hospital with a dedicated immunodeficiency center. The immunodeficiency center of our hospital registered 400 new cases of HIV/AIDS over a period of two years from February 2014 to September 2016. Institutional Ethical Clearance was obtained before commencement of the study. Out of these cases, 107 patients presented with predominantly CNS symptoms and underwent MRI brain. The predominant CNS symptoms included headache, cognitive or behavioural disorder, seizures or motor deficits. Only HIV positive patients with CNS symptoms were included in this study. The predominant exclusion criteria were standard contraindication to MRI. This study finally included 75 patients of HIV who had positive CNS imaging findings in initial MRI brain. MRI findings were correlated with laboratory results and HPE wherever possible.
MRI of the brain was performed on GE Wipro Sigma 1.5 Tesla machine, 16 Optix RF channels with Express Coils. Standard sequences (T1W, T2WI, FLAIR, Gradient, DWI and post contrast) were obtained in axial, coronal and sagittal planes. Gadodiamide (Omniscan) contrast was administered in all cases. The specific parameters applied for the study were as follows: T1WI (TR: 400, TE: 20, flip angle: 90°), PDI (TR: 1200, TE: 26, flip angle: 90°), T2WI (TR: 4000, TE: 85, flip angle: 90°), FSE T2 (TR: 2600, TE: 68, flip angle: 90°), GRE T1 (TR: variable, TE: 22, flip angle: 88°), GRE T2* (TR: 700, TE: 14.6, flip angle: 20°), STIR (TR: 2600, TE: 86, flip angle: 165°, Inversion Time (TI): 150, FLAIR (TR: 8000, TE: 113, flip angle: variable, TI: 2000). Dose of the contrast was 0.1 mmol/kg body weight. Post contrast acquisition was performed five minutes after contrast administration.
The protocols followed for this study at our HIV center was as follows:
Toxoplasmosis: Antigen and CSF PCR was done on all cases suspected to have toxoplasmosis clinically and on imaging. In cases with high suspicion of infection on imaging and negative biochemical results, treatment for Toxoplasma for four weeks was initiated. If repeat imaging, shows regression of the lesions, a presumptive diagnosis of toxoplasmosis was assumed and the patient was administered a further two weeks of medication. A total of six weeks of therapy was routinely administered for cases of toxoplasmosis.
Tuberculosis: All patients underwent CSF examination. In patients with imaging features suggestive of tubercular infection with negative CSF findings, additional scanning of the chest and abdomen with Contrast Enhanced CT (CECT) was done to rule out any tubercular foci. The findings of TB PCR and ESR were corroborated. As ATT had to be administered for one year, all efforts were made to confirm the diagnosis before initiation of ATT. Regression of clinical and imaging findings after starting of ATT was another criteria to confirm the diagnosis in cases with equivocal imaging and biochemical findings.
Cryptococcus: India ink stain and Ag test in serum and CSF were performed in all suspected cases of cryptococcosis, in addition to imaging with Contrast Enhanced Magnetic Resonance Imaging (CEMRI).
In patients with HSV encephalitis and CMV infection, PCR-based detection of DNA in CSF specimens were evaluated in addition to imaging.
MRI proved to be the main tool for diagnosing PML. Trial of HAART with symptomatic relief and regression of imaging findings were the relevant parameters relied upon.
In patients with neurocognitive symptoms, CEMRI was the main investigating tool employed.
Patients with imaging findings suggestive of CNS lymphoma underwent biopsy confirmation of the disease.
Histopathological confirmation was done in all cases of CNS lymphoma before initiation of therapy. In other cases, CSF studies and serum findings were used to substantiate our diagnosis as per the standard institutional protocol.
Statistical Analysis
Data analysis was done using the software Statistical Package for the Social Sciences (SPSS) version 21.0 Being a descriptive study means and proportions were calculated for continuous and categorical variables respectively.
Results
Of the 400 cases of HIV, 107 patients presented with CNS manifestations with predominant symptoms of headache, cognitive or behavioural disorder, seizures, motor deficits, etc. These patients were subjected to MRI brain. A total of 75 (70%) had positive MRI findings with various pathologies [Table/Fig-1].
Incidence of patient based on final diagnosis (n=75).
| Disease | Number of patients | Percentage |
|---|
| HIV Vasculitis | 01 | 1.33% |
| Tuberculosis | 27 | 36% |
| Toxoplasma | 12 | 16% |
| Lymphoma | 03 | 04% |
| IRIS | 03 | 04% |
| PML | 09 | 12% |
| HIV Encephalopathy | 15 (primary)+11* | 20% |
| HSV | 01 | 1.33% |
| Cryptococcal Meningitis | 04 | 5.3% |
| Total | 75 | 100% |
*26 had features of HIV encephalitis. 15 had only HIV encephalitis while 11 had other brain pathologies in addition to features of HIV encephalitis.
Mean age of the patients was 38.3 years (range 08-60 years) with 61 males (81.3%) and 14 females (18.7%). Out of total 75 patients, 69 patients had CD4 counts<200 CD4+T cells/μL and six patients had CD4 counts>200 CD4+T cells/μL.
There were a total of 27 cases of TB (n=27) in this study out of which 12 patients had features of tubercular meningitis. The basal exudates appeared hyperintense to CSF on T1WI, T2WI and FLAIR images with intense post contrast enhancement. A total of four patients had imaging features of communicating hydrocephalus, one patients presented with imaging findings of acute infarct in the basal ganglia and 15 cases had tuberculomas (six solitary and nine multiple). Most tuberculomas were caseating with a hypo intenserim and central hyperintensity on T2WI. All lesions had perilesional oedema and mass effect and were distributed in the corticomedullary junctions, white matter and in the cerebellar hemisphere. Conglomerate lesions were seen in four patients as multiple coalescing tuberculomas. The lesions showed ring enhancement/nodular enhancement. No case of tubercular abscess was present in our study.
A total of 26 had features of HIV encephalitis out of which 15 had only HIV encephalitis, while 11 had other brain pathologies in addition to features of HIV encephalitis [Table/Fig-2].
Associated pathologies in patients of HIV Encephalitis (n=11). The table reflects associated disorders in patient of HIV encephalitis.
| Toxoplasmosis | 02 |
| HIV vasculitis | 01 |
| Tuberculosis | 07 |
| Cryptococcus infection | 01* |
* The patient had a normal Brain MRI and the diagnosis was made on CSF studies.
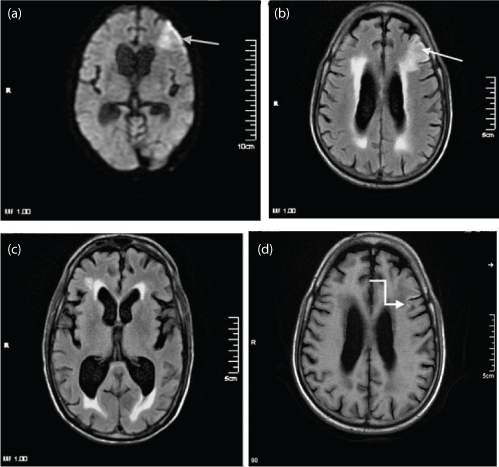
The most frequent imaging finding in patients of HIV encephalitis were diffuse brain atrophy (n=13). A total of 12 cases had extensive and symmetric involvement of the sub cortical and deep white matter appearing hyperintense on T2WI and FLAIR, typically sparing the cortical gray matter. About two patients of HIV encephalitis presented with associated pathology of Toxoplasmosis [Table/Fig-3]. Contrast enhancement and mass effect was absent in all cases. MR spectroscopy (MRS) showed decreased N Acetyl Aspartate (NAA) and increased choline (Cho) in 12 cases while it was inconclusive in rest.
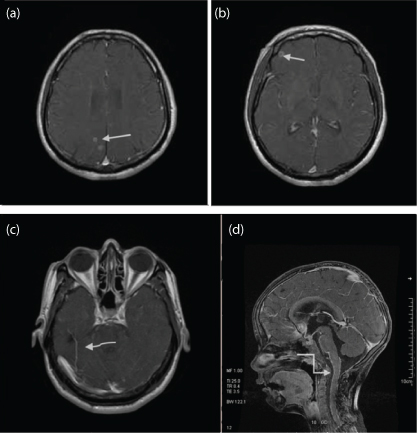
a) T1WI Sagittal MIP image of brain in a known case of HIV encephalitis, showing multiple altered signal intensity lesion involving the sub cortical and deep white matter of frontal lobe and brain stem (white straight arrow). Multiple similar lesions were seen in both cerebral hemispheres (Not shown); b) T1W Sagittal MIP post contrast images showing ring enhancement. A differential diagnosis of Toxoplasma and Tuberculomas was suspected. Follow up imaging 4 weeks after administration of Toxoplasma treatment showed reduction in size, signal intensity and enhancement pattern of these lesions (white curved arrow). Lesions are also seen in brainstem. Clinical work up for tuberculosis was negative. Final Diagnosis – Toxoplasmosis.

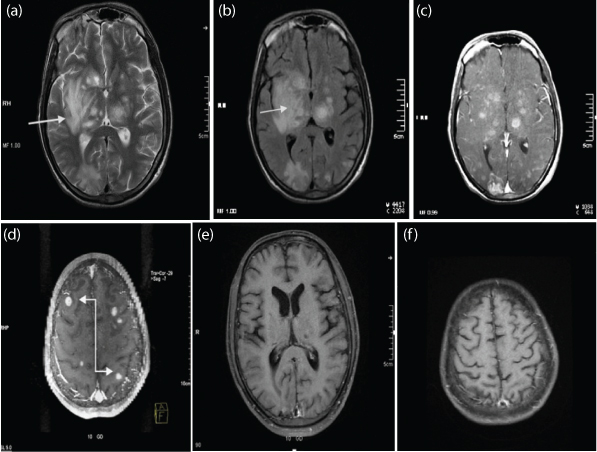
There were 12 cases of toxoplasmosis. 10 had multiple lesions [Table/Fig-4] with bilateral distribution, mainly involving the corticomedullary junctions, periventricular region, basal ganglia and thalamus. The most common location was in the basal ganglia (n=6). Most lesions showed nodular and ring enhancement. Extensive perilesional oedema was seen in all acute cases.
a,b) T2WI and FLAIR showing multiple well defined nodular lesions (White straight arrow) seen involving the subcortical and deep white matter and gray matter of the bilateral cerebral hemisphere, bilateral cerebellar hemisphere and brainstem, bilateral thalami, bilateral lentiform nucleus. Mild to moderate perilesional oedema seen. Many of them showed eccentric mural nodule; c,d) Post contrast T1WI, these lesions show peripheral ring enhancement and enhancement of the mural nodule suggestive of target appearance (White elbow arrow); e,f) Follow up after a year, post contrast imaging showing reduction post treatment. Final Diagnosis: Toxoplasmosis.
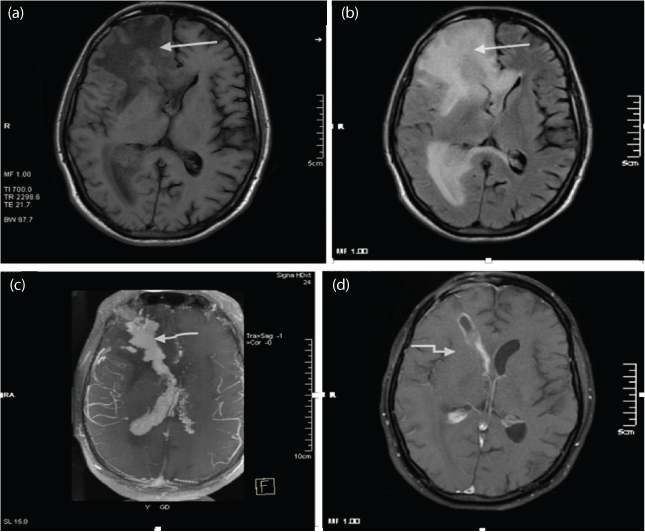
A total of three cases of PCNSL were imaged. The lesions were solitary. In one case there was involvement of sub ependyma with intraventricular extension [Table/Fig-5]. In other two patients, the lesions were predominantly involving the periventricular white matter. The lesions were isointense on T1WI and T2WI and showed intense homogenous enhancement. There was significant mass effect seen around these lesions. MRS showed increased Cho peak, reversed Cho/creatinine ratio, markedly decreased NAA and two cases showed lactate peak. The diagnosis of PCNSL was confirmed on brain biopsy in all cases.
a-c) T1WI, T2WI and T1WI Post contrast – The representative images shows a well defined intra axial lesion epicentered in the deep white matter of right frontal lobe extending from meningeal surface to the ependymal surface. The lesion is seen as hyperintense on T1WI and isointense to hypointense on T2WI (white straight arrow). The lesion showed avid enhancement on post contrast studies with associated leptomeningeal and ependymal enhancement (white curved arrow); d) 05 month follow up imaging T1WI Post contrast shows near total regession the lesion. The biopsy track is also delineated in this representatve image (white elbow arrow). Final Diagnosis: CNS Lymphoma.
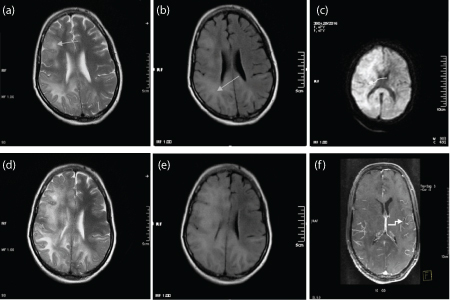
A total of nine cases were diagnosed with PML, out of which eight were male patients. In these patients, there were asymmetrical bilateral multifocal lesions involving the white matter. Involvement of subcortical U fibers was appreciated in all cases. A total of 01 patient with T cell counts of < 100 CD4+T cells/μL had similar lesions with no significant mass effect or enhancement. This case showed deterioration after initiation of HAART despite stable and rising CD4 counts. On repeat imaging, some of these white matter lesions had faint patchy enhancement with mass effect. We offered a diagnosis of PML-IRIS [Table/Fig-6].
a-c) T2WI, FLAIR and DWI. Diffuse bilateral asymmetrical altered signal intensity lesions are seen in the subcortical (Arcuate fibers) and deep white matter mainly in right fronto-parieto-temporal regions (white straight arrow) and left occipital lobe. The lesions were seen as hyper intense on T2WI and hyper intense on FLAIR images. No true restriction was seen on DWI (white curved arrow). No significant enhancement was seen on post contrast imaging (Not shown). Final Diagnosis: Progressive Multifocal Leukoencephalopathy.
HAART was started. A repeat MR brain was performed after 21 days as patient deteriorated; d,e) Repeat imaging T2WI and FLAIR showed increase in gyral swelling with subtle enhancement of the lesion and leptomeninges in post contrast; f) T1WI (elbow arrow). Final Diagnosis: Inflammatory PML.
One case of HIV vasculitis was seen in an elderly patient. The patient presented with sudden onset right hemiparesis. MRI revealed an infarct in the MCA territory showing restriction of diffusion on DWI and corresponding ADC maps [Table/Fig-7].
a-c) DWI and FLAIR– Hyper intense peripheral wedge acute ischemic infarcts seen involving the left MCA and ACA watershed territory (white straight arrow); d) Gyriform T1WI hyper intensity seen along the middle frontal gyrus, suggestive of cortical laminar necrosis (white elbow arrow). Nonspecific white matter changes and cerebral atrophy is also seen. Final Diagnosis: Acute ischemic infarct in HIV vasculitis.
There were four cases of Cryptococcus in which the MR imaging was essentially normal. All these had positive CSF culture for Cryptococcus.
Discussion
In our study, the mean age of the patients was 38.3 years (range 4–60 years). About 33% of patients were between 41-50 years. This age pattern conforms to findings of a large study in United States which revealed that persons aged 45-54 years made up the largest percentage of persons living with diagnosed HIV [7].
There were 61 (81.3%) males and 14 (18.7%) females. A study by the CDC Atlanta revealed a male preponderance likely due to higher prevalence of multiple sexual partners [7]. In our study, the higher prevalence of males is likely due to the clientele of our organisation, which is predominantly male.
HIV is the most powerful risk factor for reactivation of latent TB and in turn TB co-infection accelerates the progress of HIV infection. Approximately 5%-9% of AIDS patients develop TB with CNS infection occurring in 2%-18% of these patients [8]. The most common CNS manifestation of TB is meningitis followed by tuberculomas. On MRI, meningitis is seen as basal exudates having higher signal intensity than CSF on T1WI, T2WI and FLAIR images and enhance brightly on post-contrast imaging. Obstructive hydrocephalus and vasculitis resulting in cerebral infarctions are associated complications [9]. Whiteman M et al., found that 36% of patients in their study had cerebral infarctions following CNS TB [10]. Tuberculomas can be single or multiple, caseating or non-caseating with surrounding oedema and are often supratentorial. Non caseating granulomas appear hypo intense on T2WI and show nodular contrast enhancement while caseating have a hyperintense center with an hypo intense rim on T2WI and show ring enhancement. Tuberculous abscesses are larger in size (>3cm). On MRS they have a lipid lactate peak [2,8]. We had 27 cases (36%) of CNS TB, with most of them (n=24) presenting with imaging findings suggestive of meningitis. The imaging features and appearances of TB in this study on MRI were similar to established findings as mentioned above. However, the incidence of TB in this study is higher than reported in literature, probably reflecting the higher incidence of TB in our country.
Toxoplasmosis caused by Toxoplasma gondii parasite, is the most common opportunistic infection and the main cause of a CNS mass lesion, affecting 15%-50% of HIV patients [2,11]. Multifocal lesions are common and only 15%-20% of lesions are solitary [2,3]. On T1WI the lesions are hypointense, while hyperintensity on T1WI is suggestive of haemorrhage which differentiates it from untreated lesions of lymphoma. On T2WI, lesions show central hyperintensity (necrotising abscess) with a hypointense rim. Lesions showed nodular or ring enhancement. In addition, lesions may show an enhancing eccentric nodule, ‘target sign’, which is suggestive of toxoplasmosis. Lesions show reduced Cerebral Blood Volume (CBV) on perfusion studies. Some cases may show features of disseminated encephalitis. In the 12 (16.66%) cases of Toxoplasmosis, 10 had multiple lesions with characteristic locations, appearances and enhancement pattern as quoted in published literature [12].
HIV encephalitis and leukoencephalopathy (synonymous with AIDS dementia complex) are the result of direct effect of the HIV infection resulting in cognitive impairment [2,11,13]. Risk factors include increasing age, longer duration of HIV infection and low CD4 count cells [11,13]. It is characterised by brain atrophy disproportionate to the age and diffuse white matter demyelination. T2WI and FLAIR images initially show bilateral patchy white matter hyperintensities, which become diffusely confluent involving the subcortical and deep white matter. HIV encephalitis does not enhance or restrict, has no mass effect and spares the subcortical U fibers. These findings help to differentiate it from PML [2,14]. Features of brain atrophy and white matter changes seen in 26 cases in this study are in concurrence with the findings of HIV encephalitis.
PML is an opportunistic infection caused by JC virus in patients with CD4 counts of 50–100 CD4+T cells/μL affecting approximately 4%-5% of AIDS patients [3,15]. The spectrum includes the classic PML, inflammatory PML, JCV encephalopathy, meningitis and infection of the granular cell layer of the cerebellum [2,3]. Detection of virus in the CSF by PCR has a high specificity of almost 96% [16]. On MRI, the lesions appear hyperintense on T2WI and FLAIR, predominantly involving the subcortical and deep white matter with involvement of the U fibers and sparing the cortex. Enhancement and mass effect is seen in inflammatory PML and PML associated with IRIS. Active lesions may restrict on DWI. MRS findings are non-specific. There were 09 cases of PML in our study, with male (n=08) predominance. Imaging features of bilaterally asymmetrical white matter lesions with involvement of the subcortical U fibers seen in the study are consistent with imaging features of PML.
IRIS is seen in patients with clinical deterioration in the setting of rising CD4 counts and occurs due to restored immunity causing an exaggerated response to infectious and non-infectious antigens [17]. IRIS is most commonly associated with JC virus (PML-IRIS), TB (TB-IRIS) and fungal infection especially cryptococcus. Imaging features can have bizarre appearances with enhancing masses and mass effect [3,18,19]. In our study, one case of PML-IRIS had features of mass effect and enhancement in the white matter lesions.
CNS lymphomas (diffuse large B cell) are the second most common cause of a cerebral mass in a HIV patient seen in 2%-6% of patients. They usually present as solitary lesion, located in the basal ganglia and periventricular region with tendency to invade the corpus callosum. They appear isodense/hyper dense on NCCT, isointense on T1WI, hyperintense on T2WI and show intense post contrast enhancement [2,3,20]. Lymphomas show increased CBV and increased uptake on SPECT and PET as compared to toxoplasmosis, which is the main differential diagnosis [3,17]. PCNSL was seen in 03 cases (periventricular location) with one case showing classical imaging findings of sub ependymal involvement and intraventricular extension [Table/Fig-5]. The imaging features of the lesions were consistent with those documented in literature. The diagnosis was confirmed on brain biopsy in all cases.
The main differential diagnosis of PCNSL was neurotoxoplasmosis. Features that favoured primary CNS lymphoma include single lesion, sub ependymal spread, solid enhancement, no haemorrhage before treatment and on MRS: increased Cho. Features that favoured cerebral toxoplasmosis included multiple lesions, scattered though basal ganglia and corticomedullary junction, ring or nodular enhancement, haemorrhage occasionally occurs mostly in periphery of lesion, and on MRS: decreased Cho.
Cryptococcus is an another common opportunistic infection caused by fungus Cryptococcus neoformans in patients with CD4 counts of 50-100 CD4+T cells/μL. It has three primary manifestations in CNS: meningitis, gelatinous pseudocysts and cryptococcomas. Cryptococcal meningitis is the most common clinical manifestation of cryptococcosis and one of the most important neurological manifestations in AIDS and ranks third in frequency after primary HIV neuropathy and Toxoplasma gondii amongst infectious causes of neurological diseases in these patients. Meningeal enhancement and hydrocephalus may be seen in meningoencephalitis. Dilatation of perivascular spaces due to pseudocystsis a frequent finding and is suggestive of cryptococcal infection in a patient with low CD4 counts [21]. These pseudocysts follow CSF signal intensity on all MRI sequence. Cryptococcomas are uncommon, which can present as a mass lesion [18,21]. Four cases of Cryptococcus had positive CSF findings; however the brain imaging was essentially normal.
About 90% of congenital HIV is due to vertical transmission with infants being infected at birth or in the third trimester and accounts for 5%-20% of HIV cases [3]. Children have lower rates of infection, due to lack of prior exposure to pathogens [6]. However, they are more prone to HIV vasculopathy and stroke compared to adults. On imaging, the most consistent finding is of brain atrophy especially involving the frontal lobes. Dilated and ectatic intracranial vessels may be seen in up to 5% of cases [7,22,23]. In our study, one 6-year-old child presented with features of meningitis. On imaging faint leptomeningeal enhancement was seen [Table/Fig-8].
a-c) Post contrast T1WI- Multiple ring enhancing lesions at grey-white matter junction (white straight arrow) with evidence of meningeal enhancement (white curved arrow). Final Diagnosis: Tuberculomas with Tubercular Meningitis; d) Post contrast T1WI in a different pediatric patient shows leptomeningeal enhancement (white elbow arrow). CSF studies showed features of tubercular meningitis. Final diagnosis: Tubercular meningitis.
CMV is a herpes virus and presents in immunocompromised host as meningoencephalitis or ventriculitis. No cases were detected in our study [22,24].
Normally yellow marrow, manifested by high intensity on T1WI becomes dominant in the clivus by the age of 20 years. In AIDS patients clival marrow appears hypointense on T1WI due to haemosiderin deposition [12,23,24].
Limitation
The possible limitations of the study were firstly that histopathological confirmation was not available for all cases and the treatment was initiated on basis of imaging features and clinical suspicion in some cases. The other possible limitation is that follow up of the cases was done for over two years only which was limited to the period of the study.
Conclusion
In the complex CNS manifestations of HIV and AIDS patients, neurologic diseases are related either to direct CNS invasion by the human immunodeficiency virus or by opportunistic pathogens. Despite the increasing availability of HAART, opportunistic infections continue to afflict patients in the Indian population. Classic CNS infections in the setting of AIDS include toxoplasmosis, CNS TB, cryptococcosis, progressive multifocal leukoencephalopathy, cytomegalovirus encephalitis, and neurosyphilis. In our study, we included neuroimaging in our institutional protocol and it was a crucial component of the diagnostic workup in our study population. MRI proved to be the preferred imaging modalities for brain and spine in confirming the diagnosis due to its multiplanar capability and excellent soft tissue resolution. With characteristic imaging pattern of CNS lesions in HIV which includes but were not limited to, intracranial mass lesions, white matter disease, meningoencephalitis, vascular complications, and hydrocephalus. The imaging findings overlapped with one another but certain characteristic pattern was helpful in diagnosing a particular disease. This along with other advanced imaging technique and laboratory tests were vital to the neurology team which helped us in decision making related to a diagnostic conclusion, treatment and further workup. We conclude with a statement that findings of TB, HIV encephalopathy and Toxoplasma were the most common on evaluating HIV patients with CNS symptoms which are comparable with other studies. However, incidence of TB was higher in our population because of high incidence and prevalence of this disease in this part of the world.
Abbreviations
ADC Apparent Diffusion Coefficient
AIDS Acquired Immunodeficiency Syndrome
CMV Cytomegalovirus
CNS Central Nervous System
FLAIR Fluid-Attenuated Inversion Recovery
HAART Highly Active Antiretroviral Therapy
HPE Histopathological Examination
HIV Human Immunodeficiency Virus
IRIS Immune Reconstitution Inflammatory Syndrome
MRS Magenetic Resonance Spectroscopy
NAA N-acetylaspartate
NCCT Non Contrast Computed Tomography
PET Positron Emission Tomography
PML Progressive Multifocal Leukoencephalopathy
SPECT Single-photon Emission Computed Tomography
T1WI T1 Weighted Imaging
T2WI T2 Weighted Imaging
*26 had features of HIV encephalitis. 15 had only HIV encephalitis while 11 had other brain pathologies in addition to features of HIV encephalitis.
* The patient had a normal Brain MRI and the diagnosis was made on CSF studies.