Introduction
Many theories are suggested to explain aetiology of vitiligo, such as autoimmune, genetic and biochemical. Interleukin-8 (IL-8) is a pro-inflammatory chemokine which is evaluated in the pathogenesis of some skin diseases, like psoriasis, however, in vitiligo, few studies were reported regarding this issue.
Aim
The aim of the present study was to investigate serum level of IL-8 and IL-8 mRNA in patients with different types of vitiligo to validate its role in aetiopathogenesis of vitiligo.
Materials and Methods
This case-control study was conducted on 39 vitiligo patients and 15 age and gender matched healthy controls. The participants were selected from Dermatology Outpatient Clinic, Menoufia University Hospitals from October 2014 to October 2015. They were subjected to clinical history, examination and full general examination. Detailed dermatological examination including VASI score was applied. IL-8 serum level was measured by ELISA and IL-8 mRNA was quantitated by Real Time-PCR. The statistical analysis was done using SPSS, version 20.0. Mann Whitney U test was used to compare two quantitative not normally distributed. Chi-square test (χ2) was used to study association between two qualitative variables. Spearman correlation test was applied to assess correlation between two continuous quantitative variables. A p≤0.05 was considered significant.
Results
IL-8 serum level and mRNA concentration mean values were significantly elevated in vitiligo patients (26.25±43.28 pg/mL and 8.48±11.92 ng/mL) than controls (0.57±0.50 pg/mL and 0.60±0.32 ng/mL) (p=0.002, for both), and they showed significant positive correlation with each other (r=0.622; p≤0.001). Moreover, both of them revealed significant high values in localised vitiligo (62.20±74.39 pg/mL and 12.42±13.85 ng/mL) than generalised (18.39±29.49 pg/mL and 7.61±11.52 ng/mL) (p≤0.000 and p=0.004), respectively.
Conclusion
Serum IL-8 chemokine and its mRNA increased significantly in vitiligo patients indicating that it may have a dynamic role in vitiligo development and participate in its pathogenesis.
Introduction
Vitiligo is an acquired skin disease characterised by having white and well demarcated macules and patches of different distribution and variable sizes [1], in addition to that the melanocytes in the skin lesion are destroyed [2,3]. The disease is estimated to affect about 0.5-1% of the population worldwide, affecting men and women equally [4].
Aetiology of vitiligo is complex and multifactorial. Many theories, such as genetic, autoimmune, biochemical and adhesion defect have been suggested trying to explain its pathogenesis; however none of them succeeded in describing the full spectrum of the disease [5]. The autoimmune theory hypothesises that the destruction of melanocytes is caused by many active immunological components like memory cytotoxic T-cells and autoantibodies specific to surface or cytoplasmic antigens on melanocytes, such as γ-enolase, α-enolase and heat-shock protein 90 [6,7].
Some inflammatory cytokines were proven to be involved in vitiligo pathogenesis [8], like TNF-α [9], IFN-γ, IL-10 and IL-1β [10], IL-6 [11] and IL-8 [11,12]. Oxidative stress has a well proven role in starting the destruction of melanocytes, thereof it could be a possible theory of vitiligo [13].
IL-8 (CXCL8) is a pro-inflammatory cytokine. It is a member of CXC chemokine family that working on CXCR1 and CXCR2 receptors [14,15]. It is generated by a wide type of cells, such as neutrophils, monocytes, endothelial cells, in addition to keratinocytes [15,16]. Also, CXCR1 and CXCR2, are expressed on the surface of many cells types, like monocytes, neutrophils, CD8+ cells, immature monocyte-derived dendritic cells, natural killer cells, myeloid derived suppressor cells, keratinocytes, and melanocytes [15].
A number of different stimuli mediate the expression of IL-8, such as inflammatory signals, exposure to chemotherapy agents, hypoxia, and male sex hormones [17]. IL-8 gene expression is highly sensitive to oxidants, and anti-oxidants reactive oxygen intermediates [18], also, it is induced by TNF-α, IFN-γ, other chemokines including IL-1, and lipopolysaccharide of the bacterial wall [19,20].
IL-8 has many functions; it initiates migration of neutrophils to the site of inflammation in a process known as chemotaxis, mediates neutrophil adhesion to endothelial cells, and enhances activation of neutrophils [21].
In the field of dermatology, IL-8 is involved in the pathogenesis of many common skin diseases, such as bullous pemphigoid [22], psoriasis [23] and atopic dermatitis [24], however its role in vitiligo still needs to be elucidated.
The aim of the present study was to measure serum level of IL-8 and IL-8 mRNA in patients with different types and severity of vitiligo to shed light on its possible role in aetiopathogenesis of vitiligo.
Materials and Methods
This case-control study was applied on 39 patients with different varieties of vitiligo, in addition 15 age and gender matched healthy volunteers were chosen controls. They were selected from Dermatology Outpatient Clinic, Faculty of Medicine, Menoufia University Hospitals during the period from October 2014 to October 2015. Each individual in the study signed a written consent form approved by The Committee of Human Rights in Research of Menoufia University.
This study included newly diagnosed cases of vitiligo and old diagnosed cases stopped topical treatment for two weeks and systemic treatment for 1 month before joining the study.
Subjects having auto-immmune/inflammatory diseases, current infection or immunosuppression, pregnant or lactating women were excluded from our study.
All participants in the study were subjected to thorough history taking, stressing on onset, duration and family history of vitiligo. General clinical examination, to detect any excluding factor and dermatological examination, to evaluate the vitiligo and to assess its severity based on VASI score were also performed [25].
Measurement of the serum levels of IL-8 protein by ELISA: From each participant, 5 mL of venous blood were withdrawn under complete aseptic condition, and 2 mL of them were put in a plain tube, left for 15 minutes at room temperature to clot, centrifuged for 10 minutes at 4000 rpm and the sera were kept frozen at -80oC for measurement of IL-8 by ELISA.
Serum IL-8 was measured using Human IL-8/CXCL8 ELISA Kit (Boster Biological Technology, USA) according to the manufacturer’s instructions. This kit has sensitivity of less than 1 pg/mL with no detectable cross-reactivity with other relevant proteins.
Assessment of IL-8 mRNA by using Real Time -PCR for mRNA expression of IL-8 [12]: The other 3 mL of the collected venous blood were put in EDTA tube for extraction of total RNA from whole blood by GeneJET Whole Blood RNA Purification Mini Kit (Thermo scientific), according to the manufacturer’s protocol. The extracted RNA samples were stored at -80oC until analysis. The concentration of RNA was determined by measuring its absorbance at 260nm (A260). Absorbance readings should be greater than 0.15 to ensure significance. The ratio between the absorbance value at 260 and 280nm (A260/A280) gave an estimate of RNA purity. A260/A280 ratio greater than 1.6 was accepted. Two steps RT–PCR was done as follows: for reverse transcription step; a reverse transcriptase kit (SensiFAST cDNA synthesis kit, Bioline Reagents Ltd., UK) was used for complementary DNA (cDNA) synthesis on 2720 thermal cycler (Singapore). For cDNA synthesis, RNA (10 μL) was reverse transcribed in a final volume of 20 μL containing 1 μL of reverse transcriptase enzyme, 4 μL of 5x TransAmp buffer and 5 μL of DNase/RNase free water. The samples were incubated at 25oC for 10 minutes (primer annealing), and 42oC for 15 minutes (reverse transcription). Reverse transcriptase was then inactivated by heating at 85oC for 5 minutes. All products were stored at -20oC till the next step. For cDNA amplification: a relative quantitation of IL-8 mRNA expression normalised to the endogenous β-actin reference gene was performed by real time reverse transcription PCR (RT-PCR), using the 2x SensiFAST MSYBR® No-ROX Kit (Bioline Reagents Ltd.), on Applied Biosystems 7500 Real Time PCR System. Forward primer of IL-8: ACTGAGAGTGATTGAGAGTGGAC and reverse primer of IL-8: AACCCTCTGCACCCAGTTTTC [26]. Forward primer of β-actin primer: 5’AGTTGCGTTACACCCTTTCTTG3’ and reverse primer: 5’TCACCTTCACCGTTCCAGTTT3’. The PCR reaction mixture (final volume, 25 μL) contained 12.5 μL of 2x SensiFAST MSYBR® No-ROX Master Mix, 1 μL of each primer (Sigma), 5.5μL of DNase/RNase free water and 5 μL of cDNA. Thermo-cycling conditions were 10 minutes at 95oC, followed by 45 cycles at 95oC for 15 second, and 60oC for 1 minute. For relative quantification; The comparative Cycle threshold (Ct) method was used. Analysis was performed using Applied Biosystems 7500, software version 2.0.1. The amplification plot of IL-8 was shown in [Table/Fig-1].
Amplification Plot of IL-8 gene.
Statistical Analysis
The data were collected, entered and processed on IBMPC compatible computer using SPSS software (version 20.0) (SPSS Inc., Chicago, U.S). Two types of statistics were done; the descriptive methods (for example, mean and standard deviations for normal continuous variables, and range for non-normal continuous variables and frequencies and percentages for categorical variables) and analytic statistics: e.g., Mann-Whitney U test, a nonparametric test used to compare two quantitative not normally distributed. Chi-square test (χ2) was used to study association between two qualitative variables. Spearman correlation test (rho) to assess correlation between two continuous quantitative variables not normally distributed. A p≤0.05 was considered statistically significant.
Results
Demographic and Clinical Data
This study was conducted on 39 patients with vitiligo, they were 15 males (38.5%) and 24 females (61.5%). Their age ranged between 5 to 70 years with 40.31±18.70 years as mean. Our control group included 15 healthy volunteers, their age ranged from 7.0 to 65.0 years, with a mean value of 36.20±18.20 years. They were 5 males (33.3%) and 10 females (66.7%). They were age and sex matched with the vitiligo cases (p=0.471 and p=0.727) respectively [Table/Fig-2].
Comparison between demographic data of the studied groups.
| Parameter | Vitiligo group(n=39) | Control group(n=15) | Test of significance | p-value |
|---|
| Age (years)Mean±SDRange | 40.31±18.705.0-70.0 | 36.20±18.207.0-65.0 | Mann-Whitney =0.73 | 0.471 |
| Gender | No % | No % | χ2=0.12 | 0.727 |
| Males | 15 | 38.5 | 5 | 33.3 |
| Females | 24 | 61.5 | 10 | 66.7 |
Thirty two cases presented with generalised vitiligo (82.1%), while 7 cases with localised vitiligo (17.9%). The duration of the disease ranged between 1 month to 40 years with 12.30±10.88 years as mean value. The age of onset of the disease ranged from 4 to 59 years with 28.80±15.48 years as the mean. Family history of vitiligo was positive in 11 cases (28.2%). The severity of vitiligo ranged from 0.09 to 100% with 35.13±19.21% as the mean value [Table/Fig-3].
Characteristics of vitiligo patients.
| Parameter | Vitiligo patients |
|---|
| (n=39) | No % |
|---|
| Type of vitiligo |
| Generalised | 32 | 82.1 |
| Localised | 7 | 17.9 |
| Previous treatment for vitiligo |
| Receive ttt | 20 | 51.3 |
| Not receive ttt | 19 | 48.7 |
| Family history of vitiligo |
| Positive | 11 | 28.2 |
| Negative | 28 | 71.8 |
| Age of onset of vitiligo (years)Mean±SD Range | 4.0-59.028.80±15.48 |
| Duration of illness with vitiligo (years)Mean± SD Range | 1 month- 40 year12.30±10.88 |
| VASI score of patients with vitiligoMean± SD Range | 35.13± 19.210.09 -100 |
IL-8 (serum and mRNA) levels
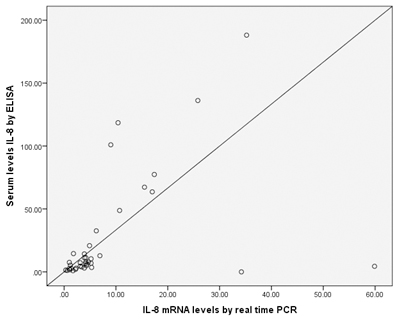
Serum IL-8 level of vitiligo group (26.25±43.28 pg/mL) was significantly higher than that of the controls (0.57±0.50 pg/mL) (p=0.002). Also, mean of IL-8 mRNA level was higher in the vitiligo group revealing 8.48±11.92 ng/mL, whereas it was 0.60±0.32 ng/mL in the control group with statistically high significant value (p=0.002) [Table/Fig-4]. Moreover, there was statistically significant positive correlation between the measured serum IL-8 level with that of IL-8 mRNA (r=0.622 and p<0.001) in vitiligo patients group [Table/Fig-5].
Comparison of serum IL-8 and IL-8 mRNA levels between the studied groups.
| Parameter | Vitiligo group (n=39) | Control group(n=15) | Mann-Whitney test | p-value |
|---|
| Serum IL-8 (pg/mL)Mean± SD | 26.25±43.28 | 0.57±0.50 | 3.03 | 0.002 |
| IL-8 mRNA (ng/mL)Mean±SD | 8.48 ±11.92 | 0.60±0.32 | 3.05 | 0.002 |
Correlation between IL-8 mRNA levels and serum levels of IL-8 in vitiligo cases.
Relationship between IL-8 serum and IL-8 mRNA levels with demographic and clinical criteria
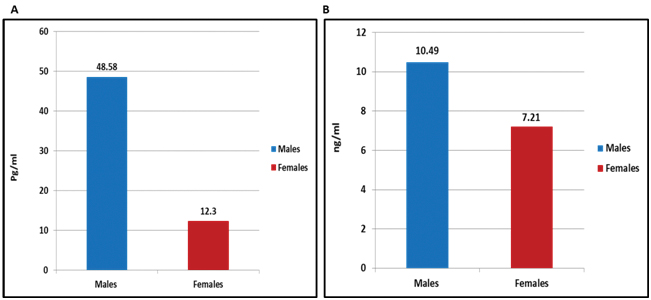
IL-8 serum and IL-8 mRNA concentration increased significantly (p=0.005 and p=0.003, respectively) in males vitiligo patients (48.58±57.98 pg/mL and 10.49±9.35 ng/mL, respectively) than females patients (12.30±22.81 pg/mL and 7.21±13.31 ng/mL, respectively) [Table/Fig-6].
a) IL-8 serum; and b) IL-8 mRNA levels in vitiligo patients in relation to gender.
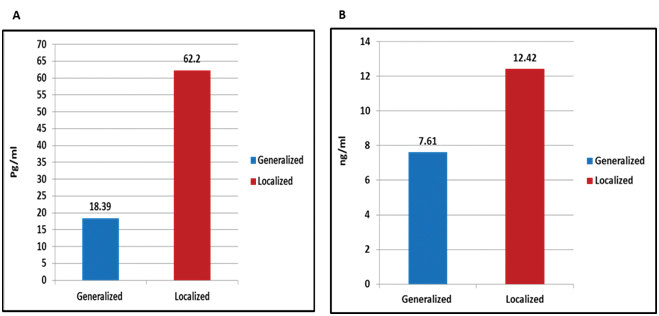
The evaluated mean value of serum IL-8 in cases with localised vitiligo was 62.20±74.39 pg/mL and in generalised vitiligo was 18.39±29.49 pg/mL, this difference was statistically significant (p<0.001). Also, there was a statistically significant (p=0.004) difference in IL-8 mRNA level between patients of localised vitiligo (12.42±13.85 ng/mL) and those with generalised (7.61±11.52ng/mL) vitiligo [Table/Fig-7].
a) IL-8 serum; and b) IL-8 mRNA levels in vitiligo patients in relation to clinical types of vitiligo.
However, the correlation between both IL-8 serum and IL-8 mRNA expression levels in the studied vitiligo cases with other studied demographic and clinical data could not reach the level of significance (data were not shown).
Discussion
To best of our knowledge, this study may be the first one that investigates both IL-8 serum level and its mRNA concentration at the same time in vitiligo patients, aiming to throw light on the possible hypothesised role of IL 8 in vitiligo development.
In the current study, we reported significantly increase in serum IL-8 level mean value in vitiligo patients than their controls (p=0.002). In agreement with our findings, Yu HS et al., revealed a significant elevation in IL-8 serum level in their studied vitiligo patients than control group [11]. Additionally Li YL et al., observed that melanocytes produce IL-8 following stimulation by anti-melanocyte IgG antibodies [27], that are present in most vitiligo patients [28].
However, Miniati A et al., reported no significant differences between vitiligo patients and controls regarding serum level of IL-8 measured by the microbead array [29]. This difference could be attributed either to small sample size in their study (15 vitiligo cases), or different laboratory method used for analysis.
In the same context, we evaluated IL-8 mRNA total amount using real time PCR. Parallel to IL-8 serum level, our result showed significantly increase in IL-8 mRNA mean value in vililigo patients than their peers (p=0.002). Likewise, Miniati A and his colleagues reported that the lesional skin of patients with new-onset and active non-segmental vitiligo showed increased IL-8 gene expression evaluated by quantitative reverse transcriptase PCR [29]. The authors suggested that in the initial stage of the disease, where melanocytes are still found in epidermis, IL-1β and TNF produced by many skin cell types have the ability to stimulate high IL-8 skin expression by melanocytes.
It was suggested that IL-8 may attract T-cells to vitiligo lesions leading to enhancement of the inflammatory reaction and melanocyte destruction [12]. Also, IL-8 is a powerful chemokine that can induce oxidative stress causing indirectly apoptosis of keratinocyte and these apoptotic cells could release increasing amounts of pro-inflammatory cytokines, such as IL-1 and TNF enhancing skin inflammation melanocyte [30]. Added to this, IL-8 may directly inhibit the growth and mediate expression of antigens on or in the cytoplasm of melanocytes [31].
Within the vitiligo patient group, evaluated serum IL-8 showed significant positive correlation with the measured IL-8 mRNA. This correlation was not reported till now, besides, it may reflect the source of serum IL-8 in vitiligo patients.
Moreover, both serum IL-8 and IL-8 mRNA were correlated significantly with male gender that could be attributable to the male sex hormones which have stimulatory effect on the IL-8 expression, resulting in high IL-8 levels in males than females [17].
Additionally, both serum IL-8 and its mRNA were higher in the localised type of vitiligo than generalised form, that could be partially explained by increased IL-8 in the early stages of the disease because of exposure to oxidative stress, initiating its occurrence, then IL-8 level was consumed and declined in the generalised vitiligo.
Limitation
Small number of cases; finally, we recommend more studies on a large scale of vitiligo patients to confirm our results. Elegant studies for evaluation of the possible use of anti-IL-8 agents as therapeutic means in vitiligo treatment program, also suggested.
Conclusion
IL-8 serum level and its mRNA expression increased significantly in vitiligo patients than their matched peers, indicating that IL-8 may have an active role in vitiligo development and participate in its pathogenesis. Moreover, from this piece of work, IL-8 may be a novel candidate that represents, in our opinion, a future therapeutic target in the pathogenesis of vitiligo.
[1]. Lotti T, Zanardelli M, D’Erme AM, Vitiligo: what’s new in the psycho-neuro-endocrine-immune connection and related treatmentsWien Med Wochenschr 2014 164(13-14):278-85. [Google Scholar]
[2]. Yaghoobi R, Omidian M, Bagherani N, Vitiligo: a review of the published workJ Dermatol 2011 38:419-31. [Google Scholar]
[3]. Kumar R, Parsad D, Melanocytorrhagy and apoptosis in vitiligo: connecting jigsaw piecesIndian J Dermatol Venereol Leprol 2012 78(1):19-23. [Google Scholar]
[4]. Ezzedine K, Eleftheriadou V, Whitton M, van Geel N, VitiligoLancet 2015 386(9988):74-84. [Google Scholar]
[5]. Begum R, Marfatia YS, Laddha NC, Dwivedi M, Mansuri MS, Singh M, Vitiligo: a complex disease and a complex approachMolCytogenet 2014 7(Suppl 1):I57 [Google Scholar]
[6]. Pichler R, Sfetsos K, Badics B, Gutenbrunner S, Berg J, Auböck J, Lymphocyte imbalance in vitiligo patients indicated by elevated CD4+/CD8+ T-cell ratioWien Med Wochenschr 2009 159(13-14):337-41. [Google Scholar]
[7]. Waterman EA, Gawkrodger DJ, Watson PF, Weetman AP, Kemp EH, Autoantigens in vitiligo identified by the serological selection of a phage-displayed melanocyte cDNA expression libraryJ Invest Dermatol 2010 130(1):230-40. [Google Scholar]
[8]. Galadari I, Serum levels of the soluble interleukin-2 receptor in vitiligo patients in UAEEur Ann Allergy ClinImmunol 2005 37(3):109-11. [Google Scholar]
[9]. Zailaie MZ, Decreased proinflammatory cytokine production by peripheral blood mononuclear cells from vitiligo patients following aspirin treatmentSaudi Med J 2005 26(5):799-95. [Google Scholar]
[10]. Grimes PE, Morris R, Avaniss-Aghajani E, Soriano T, Meraz M, Metzger A, Topical tacrolimus therapy for vitiligo: therapeutic responses and skin messenger RNA expression of proinflammatory cytokinesJ Am Acad Dermatol 2004 51(1):52-61. [Google Scholar]
[11]. Yu HS, Chang KL, Yu CL, Li HF, Wu MT, Wu CS, Alterations in IL-6, IL-8, GM-CSF, TNF-alpha, and IFN-gamma release by peripheral mononuclear cells in patients with active vitiligoJ Invest Dermatol 1997 108(4):527-29. [Google Scholar]
[12]. Toosi S, Orlow SJ, Manga P, Vitiligo-inducing phenols activate the unfolded protein response in melanocytes resulting in upregulation of IL6 and IL8J Invest Dermatol 2012 132:2601-09. [Google Scholar]
[13]. Jian Z, Li K, Song P, Zhu G, Zhu L, Cui T, Impaired activation of the Nrf2-ARE signaling pathway undermines H2O2-induced oxidative stress response: a possible mechanism for melanocyte degeneration in vitiligoJ Invest Dermat 2014 134(8):2221-30. [Google Scholar]
[14]. Baggiolini M, Clark-Lewis I, Interleukin-8, a chemotactic and inflammatory cytokineFEBSLett 1992 307(1):97-1. [Google Scholar]
[15]. Russo RC, Garcia CC, Teixeira MM, Amaral FA, The CXCL8/IL-8 chemokine family and its receptors in inflammatory diseasesExpert Rev ClinImmunol 2014 10(5):593-19. [Google Scholar]
[16]. Brat DJ, Bellail AC, Van Meir EG, The role of interleukin-8 and its receptors in gliomagenesis and tumoral angiogenesisNeuro Oncol 2005 7(2):122-33. [Google Scholar]
[17]. Waugh DJ, Wilson C, The Interleukin-8 Pathway in CancerClin Cancer Res 2008 14(21):6735-41. [Google Scholar]
[18]. DeForge LE, Preston AM, Takeuchi E, Kenney J, Boxer LA, Remick DG, Regulation of interleukin 8 gene expression by oxidant stressJ Biol Chem 1993 268(34):25568-76. [Google Scholar]
[19]. Shi Q, Wang J, Wang XL, VandeBerg JL, Comparative analysis of vascular endothelial cell activation by TNF-alpha and LPS in humans and baboonsCell Biochem Biophys 2004 40(3):289-83. [Google Scholar]
[20]. Venza I, Cucinotta M, Visalli M, De Grazia G, Oliva S, Teti D, Pseudomonas aeruginosa induces interleukin-8 (IL-8) gene expression in human conjunctiva through the recruitment of both RelA and CCAAT/enhancer-binding protein beta to the IL-8 promoterJ Biol Chem 2009 284(7):4191-99. [Google Scholar]
[21]. Qazi BS, Tang K, Qazi A, Recent advances in underlying pathologies provide insight into interleukin-8 expression-mediated inflammation and angiogenesisInt J Inflam 2011 2011:908468 [Google Scholar]
[22]. Schmidt E, Zillikens D, Pemphigoid diseasesLancet 2013 381:320-32. [Google Scholar]
[23]. Wu P, Ma G, Zhu X, Gu T, Zhang J, Sun Y, Cyr61/CCN1 is involved in the pathogenesis of psoriasis vulgaris via promoting IL-8 production by keratinocytes in a JNK/NF-κB pathwayClin Immunol 2017 174:53-62. [Google Scholar]
[24]. Bogaczewicz J, Malinowska K, Sysa-Jedrzejowska A, Wozniacka A, Medium dose ultraviolet A1 phototherapy and mRNA expression of interleukin 8, interferon γ, and chemokine receptor 4 in acute skin lesions in atopic dermatitisPostepy Dermatol Alergol 2016 33(3):170-75. [Google Scholar]
[25]. Hamzavi I, Jain H, McLean D, Shapiro J, Zeng H, Lui H, Parametric modeling of narrowband UV-B phototherapy for vitiligo, using a novel quantitative tool: the Vitiligo Area Scoring IndexArch Dermatol 2004 140:677-83. [Google Scholar]
[26]. Spandidos A, Wang X, Wang H, Seed B, PrimerBank: a resource of human and mouse PCR primer pairs for gene expression detection and quantificationNucleic Acids Res 2010 38:D792-D799. [Google Scholar]
[27]. Li YL, Yu CL, Yu HS, IgG anti-melanocyte antibodies purified from patients with active vitiligo induce HLA-DR and intercellular adhesion molecule-1 expression and an increase in interleukin-8 release by melanocytesJ Invest Dermatol 2000 115:969-73. [Google Scholar]
[28]. Kemp EH, Waterman EA, Weetman AP, Autoimmune aspects of vitiligoAutoimmunity 2001 34:65-77. [Google Scholar]
[29]. Miniati A, Weng Z, Zhang B, Therianou A, Nicolaidou E, Stratigos AJ, Stimulated human melanocytes express and release interleukin-8, which is inhibited by luteolin: relevance to early vitiligoClin Exp Dermatol 2014 39(1):54-57. [Google Scholar]
[30]. Schallreuter KU, Bahadoran P, Picardo M, Slominski A, Elassiuty YE, Kemp EH, Vitiligo pathogenesis: autoimmune disease, genetic defect, excessive reactive oxygen species, calcium imbalance, or what else?Exp Dermatol 2008 17:139-40. [Google Scholar]
[31]. Krasagakis K, Garbe C, Zouboulis CC, Orfanos CE, Growth control of melanoma cells and melanocytes by cytokinesRecent Results Cancer Res 1995 139:169-82. [Google Scholar]