Hypertensive disorders of pregnancy account for approximately 30% of maternal deaths [1]. Most of the adverse maternal outcomes are significantly more common in PE compared to other hypertensive disorders of pregnancy. Hypertension accounts for 14% of global maternal deaths and is the second most common cause of maternal mortality as per WHO report [2]. However, in developed countries like U.S. the incidence of maternal mortality due to hypertensive diseases is only 7.4% in the year 2011-2013 [3].
Severe PE and early PE account for most of the life threatening maternal complications i.e., eclamptic convulsion, HELLP syndrome, placental abruption, acute cardiovascular complications, pulmonary oedema [4-6]. Therefore, there is a need to evaluate a marker which could predict complications of PE to optimise obstetric care and timely intervention, thereby prevent morbidity and mortality in these women.
Various markers have been studied in PE and found to be raised in pre-eclamptic women ie. Corin [7]. LDH [8], leptin [9], PAPP-A [10], inhibin and activin A [11], cystatin C, beta 2 microglobulin [12], beta-trace protein [12], sFlt-1/PGF ratio [13], BNP and NT-proBNP [14-17]. Their levels have been associated with vascular, renal, cardiac and other pathological changes of PE. Despite these different potential markers for PE, the reliability of these in predicting severity and complications of pregnancy in PE has been inconsistent between different studies.
Pro-BNP is secreted by myocytes as a result of myocardial stretch and is further broken down to active hormone BNP and a biologically inactive product NT-proBNP. It modulates the cardiovascular system by limiting myocardial hypertrophy, causing peripheral vasodilatation and increasing endothelial permeability [18,19] and remains elevated for three to six months postpartum.
NT-proBNP levels are higher in pregnant than non pregnant women as a result of myocardial stretch mediated by volume overload [20,21]. This level is even higher in pregnancy complicated with PE because of increased afterload superimposed over the pre-existing volume overload and reduced metabolic clearance of NT-proBNP due to renal impairment [15]. BNP has a half-life of 20 minutes in contrast to NT-proBNP which has a half-life of one to two hours. Thus, NT-proBNP is a surrogate marker of BNP and has a wider detection rate and better biomarker for predicting mortality, morbidity and hospitalization for cardiac failure, left ventricular dysfunction and coronary artery disease [19].
We therefore, plan to evaluate plasma NT-proBNP levels in pre-eclamptic and normotensive healthy pregnant women and also to find association of plasma NT-proBNP levels with maternal complications in both the groups.
Materials and Methods
This prospective observational study was conducted from May 2015 to May 2016 in the Department of Obstetrics and Gynaecology at a tertiary care center, Delhi, India. Approval from Ethical Committee was obtained vide letter No. HRH/2015/2550 dated 29.5.15.
Sample size:
Sample size was calculated on the basis of formula: n=z2P(1-P)/d2
Where z=1.96 for α 0.05 at 95% confidence interval
P=expected proportion in population based on previous study (taken as 0.03) [22]
Prevalence of PE 3% [22]
D=allowable error taken as 0.05 (5%)
Sample size=1.96x1.96x0.03x 0.97/0.052=44.71
A total of 90 women were enrolled comprising of two groups of 45 each; Group A with PE and group B with normotensive pregnancy. Informed written consent was taken from all the participants.
Inclusion Criteria
PE was defined as per ACOG 2013 criteria [23] i.e., SBP ≥140 mmHg and DBP ≥90 mmHg after 20 weeks measured on two occasions four hours apart in a previously normotensive women + proteinuria (≥ 300 mg / 24 hour or protein: creatinine ratio ≥ 0.3 or dipstick 1+ persistent) or in absence of proteinuria and one or more of the following features: 1) Thrombocytopenia (platelets <100,000/μL); or 2) renal insufficiency (creatinine >1.1 mg/dl or doubling of creatinine in absence of renal disease); or 3) Liver involvement (elevated serum transaminase levels twice normal); or 4) Pulmonary oedema; or 5) Cerebral symptoms or visual symptoms.
PE with severe features is defined [23] as any one of the following features- BP 160 /110 mmHg or higher on two occasions four hours apart while the patient is on bed rest (unless antihypertensive treatment is initiated before this time) or thrombocytopenia (platelets <100,000/μL) or impaired liver function (elevated serum transaminase levels twice normal) or renal insufficiency (creatinine >1.1 mg/dl or doubling of creatinine in absence of renal disease) or pulmonary oedema or new onset cerebral or visual disturbances.
Exclusion Criteria
Patients with chronic hypertension, renal disease, severe anaemia, diabetes, thyroid disease, autoimmune disease, cardiac disease, multiple pregnancy, placenta previa and patients who did not give consent were excluded from the study.
Procedure
Fasting venous sample was drawn at term or just before induction of labour or in early labour in both the groups. All baseline investigations; CBC, PCV, Liver Function Test (LFT), Kidney Function Test (KFT), PT/INR, LDH, S.Creatinine along with NT-proBNP were estimated. Plasma NT-proBNP measurement was done using Roche CARDIAC proBNP kit using cobas h 232 instrument in heparinized venous blood [24]. It is an immunoassay test using gold-labelled antibodies against NT-proBNP which form a sandwich complex with it, in the blood. The sandwich complexes thus formed gives a visual signal as a red line, the intensity of which is converted to a quantitative result which is read in the display.
Urine albumin was evaluated by dipstick [23]. History, examination findings, results of biochemical investigations, maternal outcome and other associated complications were entered in a predesigned proforma. All patients were followed up for 48 hours post-delivery and any maternal complications i.e., eclampsia or any cardiovascular complication etc., that occured postpartum within 48 hours were also recorded.
Statistical Analysis
Data was analysed using Statistical Package for the Social Sciences (SPSS) software version 20.0 Unless denoted otherwise, categorical data were described as percentages and continuous data were described as means±SD or median (inter-quartile range). A student’s t-test (for data that was normally distributed) or a Mann Whitney test (for data that was not normally distributed) was applied for comparison and statistical significance. The graphical representation was done using a Box and Whisker plot to show distribution of the values in each of the groups. Association of NT-proBNP with various parameters like parity, period of gestation, systolic and diastolic BP, impending signs and symptoms, PE features was statistically determined by using ANOVA/Kruskal Wallis/Mann whitney test. Correlation of NT-proBNP levels with parameters like age and blood investigations among the two groups was statistically determined using Pearson correlation for data distributed normally and Spearman correlation for data not distributed normally.
Results
Demographic Details
Distribution of women in both the groups with regard to age, parity and period of gestation was comparable in both the groups. Significant lower values were observed in Group A for platelet count. Whereas, the values of serum bilirubin, Serum Glutamate Oxaloacetic Transaminase (SGOT), Serum Glutamic Pyruvate Transaminase (SGPT), serum Lactic Dehydrogenase (LDH), International Normalized Ratio (INR), prothrombin time and serum NT-proBNP levels were significantly higher (p-value<0.05) [Table/Fig-1].
Baseline characteristics of study population.
| Parameter | Group A | Group B | p-value |
|---|
| n (%) | n (%) |
|---|
| Age in years |
| <25 | 20 (44.4%) | 17 (37.8%) | 0.231 |
| 25-30 | 24 (53.3%) | 23 (51.1%) |
| >30 | 1 (2.2%) | 5 (11.1%) |
| Mean±SD | 25.31±3.30 | 25.91±4.67 |
| Parity Nulliparous | 26 (57.77%) | 26 (57.77%) | 1 |
| Para ≥1 | 19 (42.22%) | 19 (42.22%) |
| POG weeks |
| <34 | 5 (11.1%) | 5 (11.1%) | 1 |
| 34-37 | 16 (25.5%) | 16 (25.5%) |
| >37 | 24 (53.3%) | 24 (53.3%) |
| Blood Parameters |
| Hb (gm%) | 10.81±1.59 | 10.4±1.46 | 0.212 |
| PCV (%) | 33.86±4.94 | 33.02±4.43 | 0.339 |
| Platelets (lacs/mL) | 1.91±0.75 | 2.23±0.48 | 0.026* |
| T.Bilirubin mg/dL | 1.03±0.73 | 0.79±0.06 | 0.027* |
| SGOT (IU/L) | 50.38±52.35 | 26.67±6.39 | 0.001* |
| SGPT (IU/L) | 44.53±41.6 | 23.47±5.43 | 0.000* |
| S.Creat. (mg/dL) | 0.92±0.2 | 0.85±0.06 | 0.420 |
| LDH (IU/L) | 361.43±243.61 | 194.38±29.7 | <0.001* |
| PT (seconds) | 14.67±1.2 | 13.81±0.37 | <0.001 |
| INR | 1.08±0.13 | 1.01±0.02 | <0.001* |
| NT-proBNP pg/ML |
| Range | 60-1682 | 60-185 | <0.001* |
| Mean | 439.47±431.99 | 99.40±37.89 |
| Interquartile range | 687 | 66 |
*significant POG- period of gestation
p-value calculated by Chi-square test and Student t-test
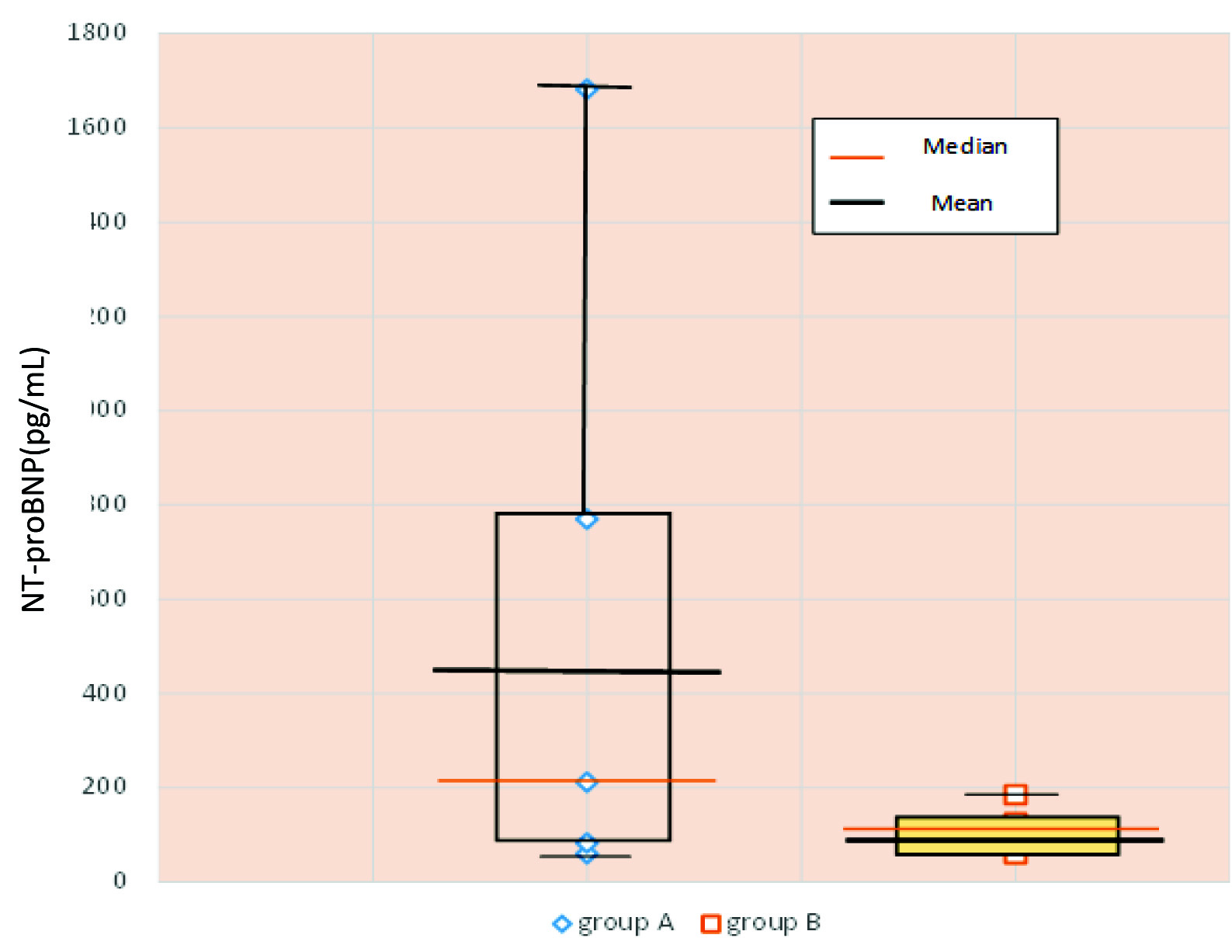
[Table/Fig-2] shows a box plot, the vertical lines showing the range of the NT-proBNP levels in both the groups. The box depicted on both the vertical lines show the central 50% of the values; between quartile 1 and quartile 3. The horizontal lines represent the mean and median values. (439.47 pg/mL and 212 pg/mL in Group A versus 99.40 pg/mL and 102 pg/mL in Group B respectively). The interquartile range (IQR) is 687 pg/mL in PE women.
Plasma NT- PROBNP levels (pg/mL) in both the groups.
NT-proBNP had a moderate correlation with some basic lab investigations i.e., SGOT, SGPT and serum creatinine and good correlation with serum LDH in our study [Table/Fig-3].
Correlation of NT-proBNP with basic investigations.
| BaselineInvestigation | Platelet | Total Bilirubin | SGOT | SGPT | LDH | S.Creatinine | PT | INR |
|---|
| p-value | 0.757 | 0.956 | 0.003 | 0.003 | 0.000 | 0.036 | 0.913 | 0.998 |
| r-value | -0.04 | 0.008 | 0.439* | 0.431* | 0.685** | 0.314* | -0.017 | 0 |
** good correlation * moderate correlation
p-value calculated by Mann-Whitney and r-value by Spearman correlation test
The association of NT-proBNP with diastolic BP, severity of PE and eclampsia was statistically significant (p-value 0.012 and 0.03 respectively) [Table/Fig-4]. NT-proBNP was significantly higher and had a negative correlation with early onset PE (p-value 0.027, r value -0.33).
Correlation of various parameters in PE group with NT-proBNP.
| S. No. | Parameter (PE group) | NT-proBNP-value | p-value | r-value |
|---|
| 1. | Onset of PEEarly <34 weeks (n=5)Late > 34 weeks (n=40) | 720.60±589.53404.74±372.22 | 0.027* | -0.33 |
| 2. | Severity- No severe features (n=12)With severe features (n=26)Eclampsia (n=7) | 257.33±278.55384.27±415.50956.71±339.56 | 0.03* | - |
| 3. | Systolic BP mm Hg140-159 (n=20)160 (n=25) | 396.2±388.88474.08±468.61 | 0.554 | 0.206 |
| 4. | Diastolic BP mmHg90-109 (n=30)110 (n=15) | 327.67±341.823663.07±513.94 | 0.012* | 0.145 |
*significant
p-value calculated by Mann-Whitney and r-value by Spearman correlation test.
In Group A 19 women had a total of 31 complications because more than one complication developed in some women. No women had any complication in Group B. Most of the complications occurred in antepartum period. Only four complications occurred in post-partum period i.e., two women had pulmonary oedema, one had CHF and one had cerebrovascular accident. Complications in Group A women occured mostly with NT-proBNP levels above 500 pg/mL. Though, some women with partial Haemolysis, Elevated Liver Enzymes, Low Platelet (HELLP) and renal dysfunction had lower NT-proBNP levels. All the cardiovascular complications in PE women occurred with NT-proBNP levels above 770 pg/mL [Table/Fig-5].
Association of Serum NT-proBNP with maternal complications.
| Maternal complications | N | Range of NT-proBNP pg/mL | Mean NT-proBNP±SDpg/mL | p-value |
|---|
| APH (Abruption) | 3 | 506-914 | 678±211.39 | 0.170 |
| HELLP (complete) | 2 | 770-867 | 853±117.38 | 0.121 |
| HELLP (partial) | 9 | 141-1457 | 783.22±535.623 | 0.009* |
| Eclampsia | 7 | 621-1682 | 956.71±339.56 | 0.001* |
| Pulmonary oedema (1case in antepartum and 2 cases in post-partum period) | 3 | 770-1405 | 997±354.08 | 0.049* |
| Congestive heart failure(occurred in post-partum period) | 1 | 770 | 770±0 | 0.394 |
| Cerebrovascular accident(occurred in post-partum period) | 1 | 823 | 823±0 | 0.314 |
| Renal dysfunction | 4 | 60-1682 | 891±662.58 | 0.155 |
| Hypertensive retinopathy | 1 | 850 | 850±0 | 0.278 |
*significant p-value calculated by Mann-Whitney test
Sensitivity of NT-proBNP at a cut off value of 500 pg/mL was 78.94% with a specificity of 90% and a positive predictive value of 83.38%. Though, its sensitivity increased to 94.47% at a cut-off level of 100 pg/mL with a negative predictive value of 92.85% [Table/Fig-6].
Sensitivity and specificity of NT-proBNP and PE related complications.
| Cut off levels of NT-proBNP pg/mL | Women with complica-tions (n) | Sensitivity | Specificity | Positive predictive value | Negative predictive value |
|---|
| >500 | 15 | 78.94% | 90% | 83.38% | 87.09% |
| >200 | 17 | 89.47% | 73.09% | 70.83% | 90.47% |
| >100 | 18 | 94.47% | 50% | 58.06% | 92.85% |
Calculated using 2 by 2 contingency table.
NT-proBNP-values were also analysed in PE women without severe features (n=12). It was observed that six women developed eight maternal complications as more than one complication developed in some women. These PE women without severe features, who developed complications had significant increased NT-proBNP than those women who did not develop any complication (p-value 0.02) [Table/Fig-7].
NT-proBNP and complications in PE women without severe features and early onset PE.
| PE women without severe features (n=12) | Early onset PE women (n=5) |
|---|
| Women who developed complications | Women who did not develop any complication | Women who developed complications | Women who did not develop any complication |
|---|
| Number of women (n) | 6 | 6 | 3 | 2 |
| NT-proBNP range pg/mL | 60-914 | 60-153 | 914-1457 | 60-175 |
| Mean NT-proBNP pg/mL | 434±307.219 | 80.66±37.54 | 1122.67±292.50 | 117.5±81.37 |
| p-value | 0.02* | 0.083 |
*significant p-value calculated using Mann-Whitney test.
Discussion
We analysed NT-proBNP in 45 consecutive pre-eclamptic pregnant women and 45 normotensive pregnant women admitted in early labour with aim to evaluate NT-proBNP in both the groups and to correlate it with maternal outcome. We also evaluated its correlation with the various clinical characteristics like gestational age of onset of PE and its severity etc.
Demographic parameters in both the groups i.e., age, parity and period of gestation were comparable in both the groups. The mean age in our study was 25.31±3.30 and 25.91±4.67 years in Group A and Group B respectively which was lower than observed by other authors [25,26]. Nulliparous women comprised 57.7% in our study comparable to study by Kristensen K et al., [12]. A higher incidence (77%) of nulliparous women in a study on critically ill pre-eclamptic women was reported by Speksnijder L et al., [27].
Serum NT-proBNP in PE group (mean 439.47±431.99 pg/mL) was significantly high compared to control group (mean 99.40±37.89 pg/mL) in our study which corroborated with the observation by Kale A et al., and Sadlecki P et al [15,17].
A strong positive correlation of NT-proBNP with various biochemical markers of severity of PE i.e., SGOT, SGPT, LDH and S.Creatinine was observed (r-value 0.439, 0.431, 0.685 and 0.314) in our study. An increased NT-proBNP may indicate a biochemical deterioration of women’s PE status and may signify the need for an earlier intervention. This observation is in corroboration with study by Cabo Fustaret MC et al., (r-value 0.375 and 0.317 for SGOT and SGPT respectively) [28].
Early onset PE women (n=5) had significantly higher NT-proBNP levels in our study. Women having severe PE, 30% have early onset PE [29]. A negative correlation of NT-proBNP (p-value 0.027, r=-0.33) with gestational age at diagnosis of PE was found in our study. This was in corroboration with studies by Moghbeli N et al., Cabo Fustaret MC et al., and Junus K et al., [16,28,30].
In our study, mean NT-proBNP levels in PE women without severe features, with severe features and eclampsia were 257.33±278.55 pg/mL, 384.27+415.50 pg/mL and 956.71+339.56 pg/mL respectively. The association of NT-proBNP with these three sets of women was statistically significant. Seong WJ et al., also found higher levels of NT-proBNP in severe PE as compared to mild PE (1766.43 pg/mL versus 214.97 pg/mL) [26]. This observation is of clinical importance and signifies that women with higher levels of NT-proBNP levels require intensive monitoring and earlier intervention than women with lower levels. However, more studies are required to obtain a cut-off level of NT-proBNP for defining PE with and without severe features.
In our study, a total of 31 complications occurred in 19 women as many women had more than one complication. Partial HELLP syndrome was the most common complication and eclampsia was the second most common complication. Similar complications have been reported in other studies, though partial HELLP syndrome has not been mentioned in other studies. Aabidha PM et al., has reported antepartum haemorrhage as the commonest maternal complication in PE (13.97%) [31]. Adu-Bonsaffoh K et al., has reported eclampsia to be the most common complication [1] whereas Yücesoy G et al., reported eclampsia and HELLP syndrome in 11% of cases each [4].
In our study, NT-proBNP was found significantly associated with partial HELLP syndrome and eclampsia (p<0.05) [Table/Fig-5]. This signifies NT-proBNP to be a marker of these important maternal complications.
Mean levels of NT-proBNP were also significantly higher in women with pulmonary oedema (997±354.08 pg/mL) than in women without it (p-value 0.049) in Group A. This was similar to the study by Seong WJ et al., [32], who found NT-proBNP levels to be significantly higher in PE women with pulmonary oedema compared to those who did not have pulmonary oedema (5587.5±2397.6 pg/mL vs 600.5±360.6 pg/mL, p=0.000). Cabo Fustaret MC et al., found a significant association of NT-proBNP with cardiac failure (p=0.001) [28]. Speksnijder L et al., also reported significant positive correlations between NT-proBNP and diastolic pulmonary pressure (p=0.005) and pulmonary capillary wedge pressure (p=0.015) [27]. Thus increased NT-proBNP is a useful marker of maternal cardio-vascular complications.
In our study increased NT-proBNP levels at term or onset of labour were associated with four postpartum complications, two cases of pulmonary oedema (mean NT-proBNP level 793±32.52 pg/mL), one case of CHF (NT-pro BNP level 770 pg/mL) and one case of cerebrovascular accident (NT-pro BNP level 823 pg/mL). Thus, it can be concluded that NT-proBNP is a useful predictor of peripartum PE related maternal complications.
In our study, NT-proBNP levels above 500 pg/mL had a good positive predictive value (83.38%) and below 100 pg//mL a good negative predictive value (92.85%) for PE related complications. Most of the complications in our study occurred with NT-proBNP levels above 500 pg/mL. We could not find any other study regarding sensitivity, specificity, negative and positive predictive values of NT-pro BNP for all the PE related complications.
PE women without severe features were further analysed in terms of developing complications. It was seen that these PE women without severe features, the mean NT-proBNP levels were significantly high (p-value 0.02) in those women who had developed complications (mean 434±307.219 pg/mL) than those who did not (mean 80.66±37.54 pg/mL). Therefore, NT-proBNP appears to be a useful marker in women who are likely to develop complications even in women who do not have clinical or lab parameter of severe features. This is a novel finding of our study and can be extrapolated that NT-proBNP can predict complications in PE women without severe features. Early interventions and referrals to higher centers of these PE women without severe features with raised NT-proBNP values can prevent these complications and hence, maternal morbidity and mortality. We could not find similar study predicting maternal complications in PE women without any evidence of clinical or biochemical parameters of severe features.
We also analysed PE women (n=5) who had early onset at <34 weeks. Four complications occurred in three women. All these three women were found to have raised NT-proBNP (range 914-1457 pg/mL) with a mean of 1122.6 pg/mL. The two women who did not develop any complication had quite low NT-proBNP (range 60,175 pg/mL) with a mean of 117.5 pg/mL. Therefore lower NT-proBNP values in early onset PE women can predict a favourable maternal outcome. PE women who had increased NT-proBNP are associated with more complications and require earlier intervention.
Limitation
Our study included the antepartum and postpartum complications which occurred within two days of delivery. More studies are required with longer follow up, so as to study the association of NT-proBNP with long term complications in pre-eclamptic women.
Conclusion
Our study clearly illustrates that NT-proBNP levels are significantly higher in pre-eclamptic women than the controls. There is statistically significant association of NT-proBNP with life-threatening maternal complications. It has a sensitivity of 89.47% at a cut off level of 200 pg/mL and a specificity of 90% at a cut off level of 500 pg/mL. An association of NT-proBNP with severity of PE and early onset of PE is seen. In PE women without severe features significantly higher levels of NT-proBNP is associated with complications. In early onset PE, lower levels of NT-proBNP have a favorable outcome than those with higher levels of NT-proBNP. NT-proBNP therefore, is a useful marker for predicting life threatening maternal complications. It should be included in routine work up of PE women especially those without severe features as increased NT-proBNP levels may identify women who need referral to a tertiary care center and early intervention. Low levels of NT-proBNP are useful in identifying women who can be given conservative treatment in early onset PE.