Iron Deficiency (ID) is common in HFrEF and Chronic Kidney Disease (CKD) patients [1,2]. It is the most frequent cause of anaemia in HFrEF, and is also very common in the absence of anaemia. For example, in Europe, approximately half of patients with Chronic Heart Failure (CHF) have ID [1], and in Asian populations ID occurs in up to 80% of patients with HFrEF [3]. ID prevalence increases with worsening renal function and/or decreasing haemoglobin (Hb) levels [4].
The pathophysiology of ID independent of anaemia is not fully understood. It has been postulated that negative consequences of ID are due to two main causes: its role in oxygen delivery via haemoglobin, and oxygen utilization resulting from its essential role in respiratory chain processes and the Krebs cycle in mitochondria [5-7]. The adverse effect of ID on mitochondrial energy production independent of anaemia has strong associations with impaired exercise performance and Health-Related Quality of Life (HRQoL), as well as increased morbidity and mortality in HFrEF patients [1,3,4,8,9].
Multiple pathways lead to ID, defined as reduced iron storage and/or transport, including poor diet, intestinal malabsorption due to bowel wall edema, polypharmacy, recent surgical interventions, and increased blood loss due to frequent use of chronic antiplatelet, anticoagulant and non-steroidal anti-inflammatory therapy [10]. Inflammation, a feature of HFrEF, is another cause of ID. Pro-inflammatory cytokines that stimulate and maintain inflammation upregulate hepcidin, which in turn downregulates the iron transport protein, ferroportin [11]. Consequently, both intestinal absorption of dietary iron and the release of iron stores from the reticuloendothelial system leads to reduced availability of iron, even when adequate iron stores are available, i.e., reticuloendothelial block [12].
There is growing evidence that correction of ID, with or without increase in Hb, can lead to a range of patient benefits in HFrEF, including New York Heart Association (NYHA) functional class, 6-minute walk test (6MWT) performance and HRQoL [13-16]. In addition, CONFIRM-HF study suggested a risk reduction for hospitalization due to worsening heart failure over a 12-month period [16], which has both clinical outcome and healthcare resource usage implications. However, long-term (over one year) efficacy and safety of IV iron therapy in HFrEF is currently unknown and no relevant ongoing trials are registered on clinicaltrials.gov.
We have previously published short-term, 6-month results of a small, single-center study investigating the effects of IV iron on N-terminal pro-brain natriuretic peptide (NT-proBNP) and inflammatory status in anaemic patients with HFrEF and moderate renal impairment [14]. These patients were observed to have an improvement in Left Ventricular Ejection Fraction (LVEF), NYHA functional class, exercise capacity, renal function and HRQoL. Here we report the results of a 5-year follow up study evaluating the impact of treatment on survival and hospitalization, as well as iron status and HFrEF symptom severity.
Materials and Methods
Study design: This is a randomized controlled pilot study with a 5 years follow up. The enrolled patients were not a specific or selected group. They were the consecutive patients from the general population that spontaneously consulted the outpatient’s office of the cardiology section at the Hospital Alemán, Buenos Aires, Argentina. The first period of this study was according to design and treatment protocol for the original, 6-month, double-blind, placebo-controlled trial as described previously [14]. Subsequently, we set up a follow-up study to look at survival, hospitalizations, iron maintenance, HFrEF and CKD symptoms up to 5 years after the start of the original trial. The trial is registered at ClinicalTrials.gov: NCT02392910.
Ethics approval was received from the Institutional board and patient consent was sought for their ongoing participation in the follow-up phase. Due to the logistical constraints of maintaining double-blinding per the original study protocol for this extended period of time, the continuation phase was performed according to an open label protocol.
The inclusion criteria for the original trial were a confirmed CHF diagnosis with reduced LVEF (≤35%), NYHA functional class 2-4, with ID (serum ferritin <100 ng/mL and/or transferrin saturation (TSAT) ≤20%) and anaemia (Hb <12.5 gm/dL for men or <11.5 gm/dL for women) [14].
The exclusion criteria were: a) haemodialysis; b) anaemia not due to iron deficiency; c) NYHA functional class I; d) history of allergy to the iron supplements; e) acute infections known in the 4 previous weeks; f) neoplasm; g) chronic digestive diseases; h) thyroid diseases; i) congenital cardiopathies; j) receiving iron supplements and or erythropoiesis-stimulating agents (ESAs) in the 4 previous weeks; k) history of hospitalization during the four weeks before enrollment into the trial.
In the original study the patients were randomized to receive 200 mg iron weekly as IV iron sucrose (Venofer®, Vifor International, St. Gallen, Switzerland) for five weeks up to a total dose of 1000mg iron, or placebo, as an isotonic saline solution [14]. Both treatment and placebo groups had optimized background CHF therapy according to the hospital’s standard procedures. Patients were monitored via follow-up visits every six months for iron level testing and other assessments (see below). At each visit, patients saw the same physician.
Treatment: Following the initial six-month study, patients consenting to participate in the follow-up study continued to be monitored and where necessary treated per the original randomization for up to 5 years. Patients randomized to receive IV iron sucrose in the original study received additional doses of 200 mg iron (diluted in 200 mL isotonic saline solution and administered over 60 minutes) when Hb decreased below 11 gm/dL or TSAT fell to 20% or less. Patients in the control group did not receive IV iron, oral iron or placebo injections after the end of the six-month study period. The indication of blood transfusion was only for patients from both groups who presented Hb below 9 gm/dL at any time of the follow up period. Both groups continued to receive optimized HFrEF therapy throughout the study and no erythropoietin was used at any time.
Endpoints and assessments: The primary endpoint for this study was overall survival at five years after the start of treatment. The main secondary endpoint was the number of hospitalizations (all causes and related to HFrEF) during the five-year follow up. Other measurements included assessment of iron status, review of vital signs, NYHA functional class, 6-minute walk test (6MWT), transthoracic echocardiogram and collection of blood samples for analysis of hematological and biochemical variables, including renal function. The details of these tests and instruments were described in the original publication [14].
Statistical Analysis
The statistical analyses were processed through GraphPad Prism version 6.0 (GraphPad Software, Inc. San Diego, CA). For parameters with a Gaussian distribution, comparisons were carried out using an unpaired t-test between groups and a paired t-test within each group. For those parameters with a non-Gaussian distribution, Mann-Whitney tests between groups and the Wilcoxon matched pair tests within each group were used. The survival time was analysed by Kaplan-Meier curves and a log rank (Mantel-Cox) test was conducted to evaluate possible differences between groups regarding the survival time. Values were expressed as mean ± Standard Deviation (SD), and p<0.05 was considered statistically significant.
Results
Patient disposition and demographics: In the original six-month treatment study, as previously published [14], a total of 40 patients meeting the inclusion criteria were recruited and randomized to receive IV iron sucrose or placebo, 20 patients in each group. At the five-years follow up, 16 patients in the iron sucrose group and nine patients in the control group were available for evaluation and data from all remaining patients were included in the analysis [Table/Fig-1].
Patient disposition throughout the study.
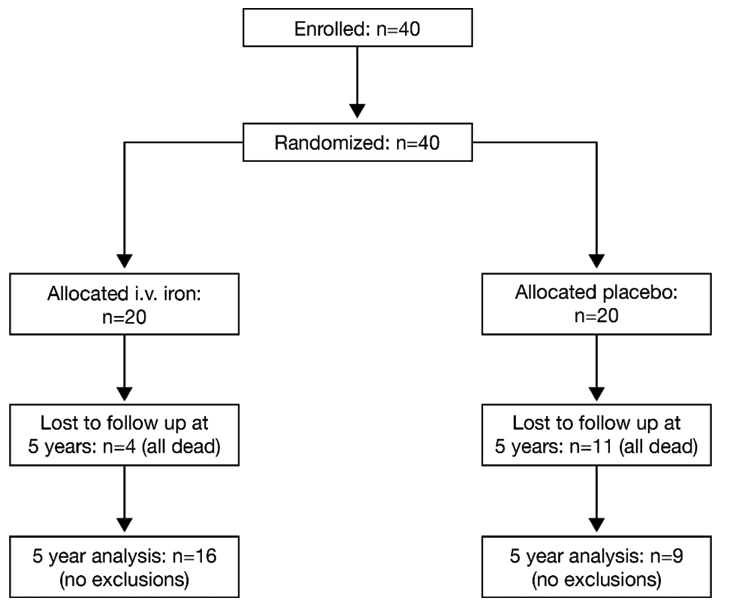
The baseline demographics for the patients in this study were published in the original report [14] and they are reproduced here [Table/Fig-2]. Both groups were well matched, with no clinically or statistically significant differences in key variables. No patients received Cardiac Resynchronization Therapy (CRT) or implantable cardioverter-defibrillator device throughout the study.
Baseline demographic characteristics, co-morbidities and heart failure etiology of the two groups at baseline.
| Variable | Control group* n=20 | Iron sucrose group* n=20 |
|---|
| Age (years old) | 74 ± 8 | 76 ± 7 |
| Male/Female | 12/8 | 13/7 |
| Diabetes | 4 | 5 |
| Coronary artery disease | 13 | 12 |
| Cardiomyopathy | 4 | 5 |
| Arterial hypertension | 3 | 2 |
| Aortic valve disease | 0 | 1 |
| Atrial Fibrilation | 4 | 4 |
| BMI (kg/m2) | 29.0±3.4 | 28.7±3.3 |
| Heart rate (beats/minute) | 80.5±8.1 | 78.7±8.2 |
| Systolic blood pressure (mm Hg) | 138.8±8.3 | 139.7±8.2 |
| Diastolic blood pressure (mm Hg) | 73.4±7.5 | 74.4±9.6 |
| Hb (gm/dL) | 10.2±0.5 | 10.3±0.6 |
| Ferritin (ng/mL) | 70.6±21.4 | 73.0±29.9 |
| TSAT (%) | 0.20±0.01 | 0.20±0.01 |
| NT-proBNP (pg/mL) | 267.5±114.9 | 255.9±124.6 |
| C-reactive protein (mg/L) | 6.6 ± 4.3 | 6.1±3.8 |
| CrCl (mL/minute) | 37.7±10.2 | 39.8±10.1 |
| EF (%) | 30.8±1.7 | 31.3±3.7 |
| NYHA functional class | 2.9 ± 0.6 | 2.9±0.7 |
| MLHFQ score | 58.0±6.0 | 60.0±5.0 |
| 6MW test (m) | 190.7±56.1 | 192.3±60.9 |
| Number of hospitalizations | 0 | 0 |
* Data are reported as mean ± SD
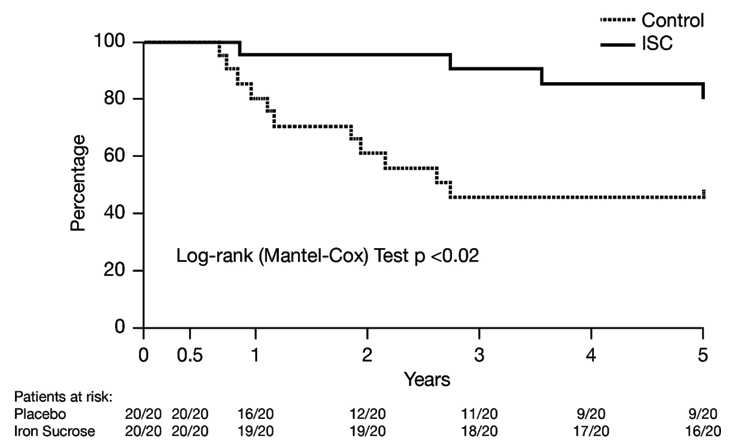
Effect of IV iron sucrose on overall survival (primary endpoint): There were no deaths during the initial treatment period. However, by third year, survival in the control group had fallen to 50%, remaining stable thereafter [Table/Fig-3]. Among patients treated with IV iron sucrose, survival was 80% after five years (relative risk ratio = 0.49; 95% confidence intervals: 0.27, 0.90; p = 0.048 versus placebo). Total deaths in control group were 11 (due to any cardiovascular CV reason= 6, and due to worsening HFrEF= 5), while in iron sucrose group were 4 (due to any CV reason= 2, and due to worsening HFrEF= 2).
Effect of intravenous iron sucrose on overall survival over 5 years.
Effect of IV iron sucrose on hospitalization: No patients were hospitalized at the start of the treatment. Throughout the study, there were fewer hospitalizations among patients treated with IV iron sucrose compared with those in the control group [Table/Fig-4]. For example, in the first 12 months, only 10% of patients receiving iron were hospitalized, compared with half of the control group (p <0.01). The Odd ratio (OR) for hospitalization in the first year was 14.167 (95% CI: 2.386, 84.107) between groups.
Effect of intravenous iron sucrose on first time hospitalizations over 5 years.
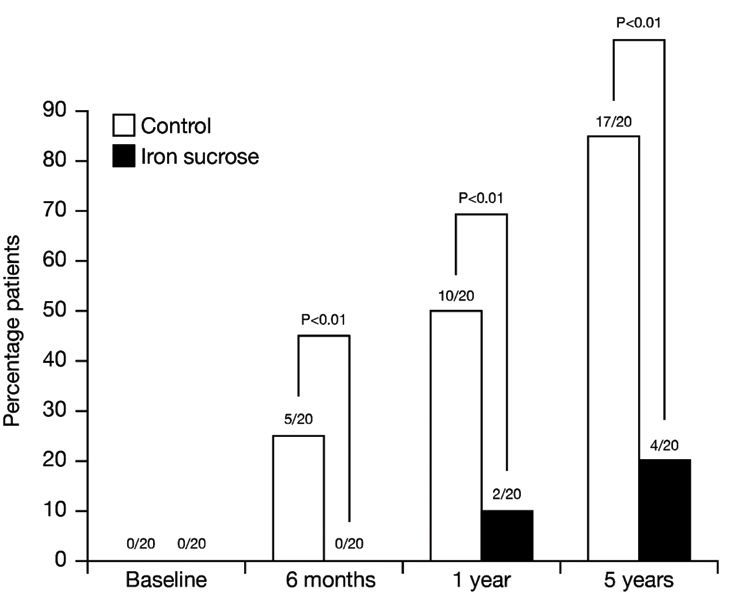
After five years, 85% of patients in the control group had been hospitalized, more than four times that among iron-treated patients (p < 0.01). Hospitalizations were related to HFrEF in all but two cases, one per group, which were due to dehydration. The hazard ratio for the hospitalization was 4.967 (95% CI: 2.086, 11.83) for the control group, compared with 0.2013 (95% CI: 0.0845, 0.479) for the iron sucrose group.
Multiple hospitalizations were only observed in the placebo group: 9/17 was hospitalized once and 6/17 and 2/17 required 2 and 3 admissions, respectively. The total number of hospitalizations in the control group was 27 versus only 4 in the group treated with IV iron.
Effect of IV iron sucrose on iron parameters and anaemia: During the original six month trial, patients in the IV iron group received 1000 mg iron per the protocol. Based on iron status, no patients required iron in the subsequent six months. From second year to fifth year, patients required an annual mean (±1 SD) of between 165 ± 190 mg and 274 ± 202 mg iron (range: 0 to 600 mg per patient per year). There was no trend in iron dosing associated with time [Table/Fig-5].
Iron administration in the follow-up period (results from intravenous iron group only).
| 12 month study period | Total* |
|---|
| Year 1* | Year 2 | Year 3 | Year 4 | Year 5 |
|---|
| Iron (mg) |
| Mean | 1000 | 274 | 244 | 165 | 188 | 2,019* |
| SD | 0 | 202 | 201 | 190 | 186 | 380* |
| Min | 1000 | 0 | 0 | 0 | 0 | 1,360* |
| Max | 1000 | 600 | 600 | 600 | 600 | 2,946* |
| Additional doses |
| 1 | - | 7 | 6 | 5 | 6 | 24 |
| 2 | - | 5 | 6 | 3 | 3 | 17 |
| 3 | - | 3 | 1 | 1 | 1 | 6 |
| 4 | - | 0 | 0 | 0 | 0 | 0 |
| >5 | - | 0 | 0 | 0 | 0 | 0 |
* including the 1000 mg administered per original protocol; no iron administered between months 7 and 12 in the first year.
Following the initial 1000 mg iron, 19 out of 20 patients required at least one subsequent dose of IV iron (iron sucrose) between years second and five, and the average time to additional iron treatment was 14.5 ± 2.7 months (range: 14 months, 23 months). As shown in [Table/Fig-5], throughout the study most patients required only one or two doses per year, and none required 4 or more.
Throughout the follow up period, iron treatment resulted in statistically significant increases in % TSAT (p<0.01 versus control group; [Table/Fig-6a]). Further, all TSAT values in the iron-treated group increased to at least 20%, the pre-defined target for iron correction. Likewise, there were statistically significant increases in serum ferritin at 6, 12 and 24 months after initiating treatment in the iron sucrose group compared with the control group (p<0.01; [Table/Fig-6b]). However, this difference was not observed at 36, 48 and 60 months.
Effect of intravenous iron sucrose on iron deficiency as determined by transferrin saturation (TSAT) percentage: (a), serum ferritin levels; (b) and haemoglobin levels; (c) over 5 years.
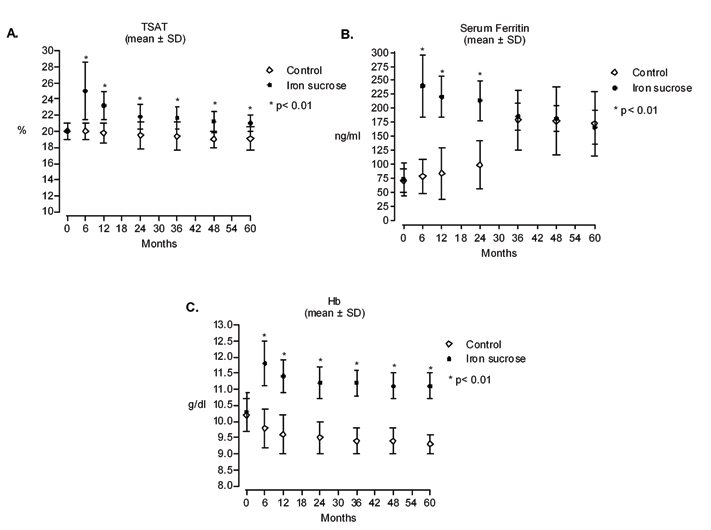
At baseline, all patients had ID anaemia [14]. Statistically significant improvements in Hb levels as well as in hematimetric parameters in iron-treated patients were seen throughout the study versus the untreated group (p < 0.01; [Table/Fig-6c,7]. Over the course of the study, mean Hb levels showed a decline from baseline in the placebo group, whereas in the iron sucrose group, Hb levels were greater than baseline at each time point.
Effect of intravenous iron sucrose on iron deficiency as determined by hematimetric parameters over 5 years: a) Mean Corpuscular Volume (MCV); b) Mean corpuscular haemoglobin (MCH); and c) Mean corpuscular haemoglobin concentration (MCHC).
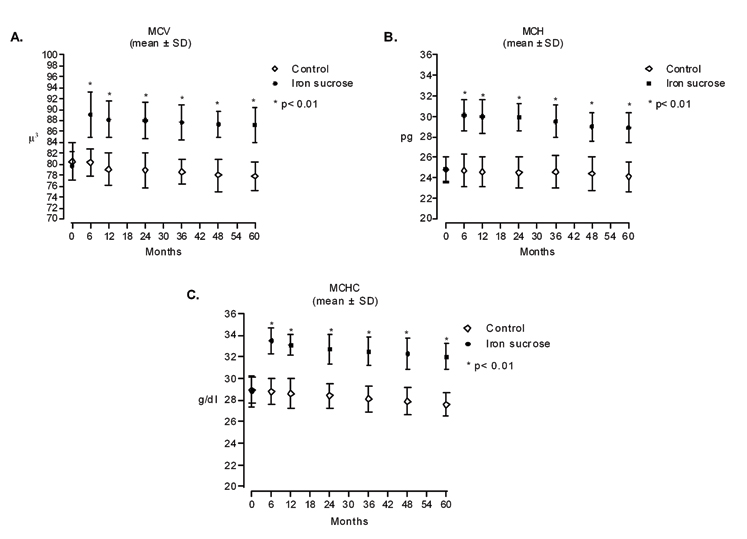
When other definitions of anaemia were considered (Hb< 11 gm/dL and Hb< 12 gm/dL, both genders), most patients remained anaemic throughout the study, although the more stringent definition of anaemia (Hb< 11 gm/dL) appeared to favour the iron sucrose group. In the control group, 16/16 patients at first year, and 9/9 patients at 5 years had Hb < 12 gm/dL. In the iron sucrose, 13/19 at 1 year and 16/16 at 5 years had Hb < 12 gm/dL. However, for Hb < 11 gm/dL, there was 15/16 at first year and 9/9 at fifth year in the control group, compared with 5/19 at first year and 9/16 at fifth year in the iron sucrose group. It worth mentioning no patient received blood transfusion throughout the study.
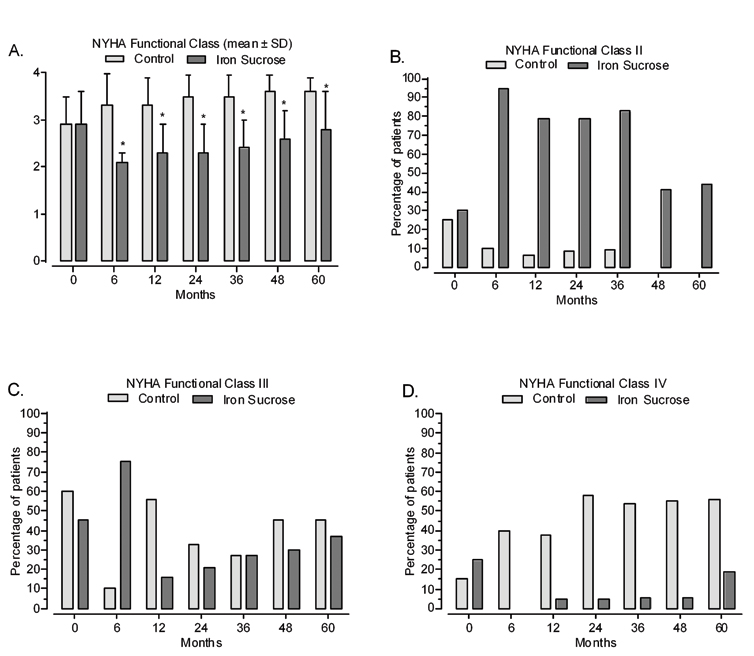
Effect of IV iron sucrose on cardiac, inflammatory and renal parameters: NYHA functional class indicates HFrEF severity. At baseline, most patients were in classes 2 or 3 [Table/Fig-8]. After 6 months, there was a statistically significant decrease in mean NHYA functional class among iron-treated patients (p < 0.01 versus control group) [14]. This improvement was maintained throughout the follow up period [Table/Fig-8]. Similar effects were seen in the percentage of patients in each NYHA functional class [Table/Fig-8]. At baseline, the placebo and treatment groups were similarly distributed (mostly NYHA functional class II and III, as shown in [Table/Fig-8 (panel b and c)]. Over the course of the study, in the placebo group, most patients were in NYHA functional classes III and IV, whereas in the iron sucrose group, most patients were in classes II and III, the majority being in class II throughout [Table/Fig-8].
Mean values and changes in NYHA class distribution from baseline throughout the study: a) NYHA class mean values; b) NYHA class II; c) NYHA class III; d) NYHA class IV. (* p< 0.01).
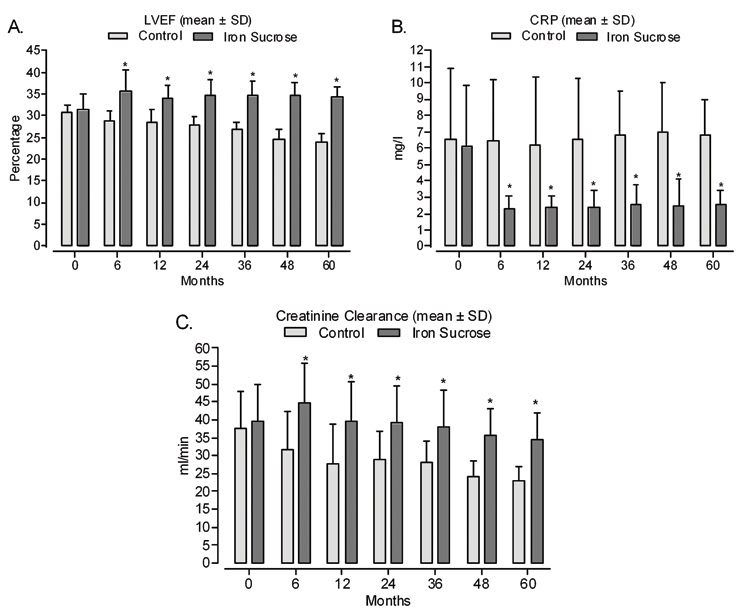
Likewise, cardiac function (% LVEF) was greater among patients receiving iron sucrose compared with the control group [Table/Fig-9a]. On the other hand, there was no overall effect of iron treatment on BMI or blood pressure, although there is an indication that heart rate lowering tended to be greater in the iron sucrose group versus the placebo group [Table/Fig-10].
a) Left ventricular ejection fraction (%); b) C-reactive protein (CRP) and c) Creatinine clearance baseline throughout the study. (* p< 0.01).
Effect of intravenous iron sucrose on BMI, heart rate and blood pressure.
| Treatmentgroup | Time after first intervention |
|---|
| 0 months | 6 months | 1 year | 5 years |
|---|
| BMI (kg/m2), | Control | 29.0±3.4 | 28.5±3.2 | 28.6±3.0 | 28.2±3.1 |
| mean±SD | Iron sucrose | 28.7±3.3 | 26.7±2.8 | 27.9±2.9 | 28.0±3.2 |
| Heart rate (BPM), | Control | 80.5±8.1 | 76.3±10.4 | 75.3±9.7 | 72.0±8.5 |
| mean±SD | Iron sucrose | 78.7±8.2 | 67.5±5.9 | 66.6±3.8** | 68.1±3.5 |
| Systolic BP (mm Hg), | Control | 138.8±8.3 | 134.3±7.3 | 132.4±8.1 | 136.1±5.0 |
| mean±SD | Iron sucrose | 139.7±8.2 | 135.7±6.1 | 135.2±5.8 | 134.5±5.6 |
| Diastolic BP (mm Hg), | Control | 73.4±7.5 | 70.2±6.6 | 70.0±6.4 | 67.2±7.0 |
| mean±SD | Iron sucrose | 74.4±9.6 | 70.1±8.3 | 68.3±7.1 | 67.0±7.1 |
**p<0.01 vs control at same time point
C-reactive protein (CRP), a marker of inflammation, was statistically significantly reduced in the iron sucrose group compared with the control group throughout follow up (p < 0.01; [Table/Fig-9b]).
As shown in the original publication, renal function (evaluated as creatinine clearance) was improved after 6 months of iron treatment compared with that in the control group [14]. During the follow-up period (12 months to 60 months), renal function declined in both groups. However, the difference between groups favouring iron treatment remained statistically significant throughout the follow up period (p< 0.01; [Table/Fig-9c]). At the end of the study, creatinine clearance had fallen by 37.2%, from 37.7 mL/min at baseline to 23.7 mL/min after 60 months, compared with a 12.9% decrease in the iron group, from 39.8 mL/min at baseline to 34.7 mL/min after 60 months.
Safety: From a safety aspect, IV iron sucrose was well tolerated. There were 3 adverse events reported in the placebo group over the course of the study: headache, nausea and abdominal pain (all n=1). In the iron sucrose group there were 5 adverse events reported: headache (n=1), rash and nausea (both n=2). No severe or significant side-effects were observed.
Discussion
There is growing evidence that IV iron therapy can help reduce HFrEF symptom severity, improve HRQoL and physical exercise capacity, and may lead to increased survival and decreased numbers of hospitalizations [13,14,17-19]. In this pilot 5-year follow up study the longest period of study of patients with HFrEF, ID anaemia and impaired renal function treated with IV iron – we found a statistically significant increase in survival (p=0.048) and a decrease in hospitalizations (p<0.001) versus placebo. It is necessary to highlight that all patients in both groups in the present study received standard treatment for HFrEF at the highest tolerated doses of the corresponding medication available at the moment of the study. The improvements observed at 6 months [14] in cardiac variables, HFrEF parameters and renal function among the patients with HFrEF, ID anaemia and CKD, who received IV iron, were also sustained throughout the follow up period as represented in [Table/Fig-9].
Since publication of the first phase of our study [14], evidence for the benefits of IV iron in patients with HFrEF and ID has grown and latest European CHF management guidelines recommend assessment of iron parameters and suggest consideration of IV iron in cases of ID [20]. Similar recommendations feature in Australian and Brazilian guidelines [21,22]. This represents a clinically important development in the management of patients with HFrEF and ID [20]. However, there is an ongoing need for long-term data for IV iron. Oral iron represents an alternative to IV formulations. However, there is a lack of data from large, controlled studies of oral iron in this setting and such formulations are not included in this discussion.
To date, most clinical investigations of the benefits of IV iron in HFrEF have focused on disease severity, physical capacity and HRQoL, but trials have been of too short a duration to evaluate survival. A recent meta-analysis of randomized controlled trials (treatment durations of 5 to 36 weeks) showed no statistically significant effect of IV iron on all-cause and CV death in patients with HFrEF and ID, with or without anaemia [17]. However, there was a non-significant trend favouring IV iron over placebo. Similar results were obtained in a recent editorial accompanying publication of the CONFIRM-HF trial [16]. Ours is the first long-term study to evaluate the relationship between IV iron treatment and survival in patients with HFrEF, ID anaemia, CKD who received standard treatment for HFrEF. Although the patient numbers are small and the trail was conducted in a single center, we demonstrated a statistically significant. Appropriately powered, multi-center, randomized trials are required to determine whether our results can be replicated.
HFrEF causes a substantial financial burden to healthcare systems, accounting for between 1% and 2% of healthcare expenditure in western countries [23], with all-cause, per-person costs for HFrEF ($44,387 per year) exceeding those for chronic obstructive pulmonary disease ($27,137 per year), coronary artery disease ($30,380 per year) and diabetes ($23,834 per year) in the US [24]. Inpatient costs are an important driver of total healthcare costs [25]. Therefore, reducing hospitalizations would seem to be a logical target to reduce the economic impact of HFrEF. Two meta-analyses have demonstrated that IV iron can help statistically significantly reduce the number of HFrEF-related and all-cause hospitalizations by between 50 and 65% after short-term treatment [17,26]. Our results indicate that these early, sizeable reductions in hospitalizations are maintained over a 5-year period, and that fewer patients required repeated hospitalizations when receiving iron compared with placebo. This could translate into substantial cost savings, potentially amounting to tens of thousands of US dollars over the patient’s lifetime. Furthermore, it is of great importance to highlight that patients in both groups in the present study were on the highest tolerated doses of medication for HFrEF available in the pharmaceutical market at the time the study was performed.
The pathophysiology of ID in relation to the effects on HFrEF symptoms, renal function, Health-related Quality of Life (HRQoL) and mortality is not fully understood. However, there is a growing consensus that these effects are largely independent of erythropoiesis and that maintaining biologically available iron stores can be an important part of HFrEF management [1,13,15,16,19,27-29]. Correcting TSAT was associated with survival and hospitalization benefits as well as other parameters. As expected, in the present study, serum ferritin levels were increased in those patients who were on IV iron treatment, and remained at recommended level (range 240.4 ng/mL to166.0 ng/mL) for patients with ID anaemia, HFrEF and CKD treated with IV iron, throughout the follow up period. Notably, while a statistically significant increase (p<0.01) in serum ferritin in the iron sucrose group with respect to the control one was observed at 6, 12 and 24 months [Table/Fig-6b], this difference was not found at 36, 48 and 60 months. The reason for this was a continuous ascending level of serum ferritin in such patients [Table/Fig-6b]. Although serum ferritin is highly linked to iron metabolism, it is also a recognized acute phase reactant and can be elevated in conditions due to inflammatory processes or worsening disease [29]. Such is the case of patients in the control group in the current study, who showed overt inflammation and aggravation of their HFrEF condition as reflected by the worsening NYHA functional class distribution [Table/Fig-8], the decline in LVEF [Table/Fig-9a]. as well as in renal function [Table/Fig-9c], together with significant higher CRP levels [Table/Fig-9b], with respect to the iv iron group. Taking into account that serum ferritin could be a confounder laboratory tool in this “clinical scenario”; TSAT may be a more reliable marker than serum ferritin of available iron stores. It is worth mentioning that findings in the present study are in concordance with some recent reports in which the authors found that ferritin was not associated with the risk of all-cause, cancer, or cardiovascular mortality in men or postmenopausal women aged 50 years or older while TSAT was inversely associated with all-cause, cancer, and CV mortality. Moreover, these results appear to show that a moderately high TSAT may be protective against oxidative stress. These findings may be helpful in understanding the association between body iron and chronic disease which is related to oxidative stress [30]. Additionally, Stack AG et al., demonstrated a significant and independent relationship between TSAT with total and CV mortality [31]. The pattern of association was j-shaped with significantly higher mortality for subjects with TSAT values <23.7% and higher than 31.3%. Both low and high TSAT ratios were significantly and independently associated with increased total and CV mortality. The optimal TSAT ratio associated with the greatest survival was between 24% and 40% [31].
In the current study, we observed at least some degree of correction and preservation of appropriate Hb levels as well as other hematimetric parameters [Table/Fig-6c,7] with IV iron only, although Hb levels mildly declined over the course of the study. However, many patients remained anaemic throughout the study. This fits well with the hypothesis that pathophysiologic effects of IV iron involve other mechanism, including muscular and cardiac effects as described above. Further evidence for the importance of non-hematopoietic mechanisms can be drawn from the RED-HF study of Erythropoiesis Stimulating Agents (ESAs) in HFrEF patients with anaemia but without ID [32]. The study demonstrated rapid correction of anaemia without any benefits on morbidity and mortality [32]. Hence, ID seems to be the actual problem in this group of patients.
As it mentioned, ID is associated with multiple health problems, including cardiovascular disease. Experimental data reported important ultrastructural myocardial changes (mitochondrial swelling, and abnormal sarcomere structure) in rats fed with an iron-deficient diet [33]. Cytochrome c release was significantly enhanced in iron-deficient rats. Protein expression of endothelial Nitric Oxide Synthase (eNOS) and inducible Nitric Oxide Synthase (iNOS), and protein nitrotyrosine formation were significantly elevated in cardiac tissue or mitochondrial extraction in these animals. Additionally, a significant up regulated NADPH oxidase, caveolin-1 and RhoA expression were also detected in ventricular tissue. Therefore, we can speculate that IV iron therapy in those patients with absolute or relative ID may provide an additional benefit beyond improving Hb level. In line with this concept, recently, our group has reported functional alterations (evaluated variables by echocardiography and Doppler tissue), morphological changes (extracellular expansion and fibrosis in left ventricle and interventricular septum) and biochemical disturbances (increased oxidative and nitrosative stress parameters, a significant abundance in tissue of phosphorylated NF-κB65, higher tumour necrosis factor-α expression in myocardium together with an increase in the repair process markers heat shock protein 27 and 70 in heart of animals fed by ID diet [34]. Interestingly, these alterations were partial or totally reversed after IV iron therapy. The positive effects of IV iron treatment were evident in the heart even though anaemia was not fully corrected. These findings suggest IV iron treatment is able to restore the function of iron as an essential component of various enzymes, thus normalizing the cellular iron homeostasis and restoring the cellular redox-balance, which contribute to preserve heart tissue [34].
Safety has been raised as a potential concern for IV iron treatment, in particular the issue of oxidative stress [35]. However, recent pre-clinical and clinical trials of new generation iron products, including our study of iron sucrose, have not identified any clinically significant safety issues related to oxidative stress in patients with HFrEF [13-16,35].
Perspectives
Clinical competencies: Our results suggest that patients with stable HFrEF who also have ID and anaemia (identified by low% TSAT and low Hb, respectively), and impaired renal function, may benefit from initial repletion with IV iron followed by ongoing management of iron levels over five years. TSAT and Hb tests are routine, inexpensive laboratory assays in the majority of cases, and offer the chance to identify patients who might experience reduced HFrEF symptom severity, improved exercise capacity and greater HRQoL after IV iron sucrose, and who may benefit from an increased chance of five-years survival and reduced need for costly hospitalizations during that time.
Translational outlook: As the evidence base for the benefits of iron repletion in patients with HFrEF and ID grows, due to increasing numbers of randomized, controlled trials as well as single center studies such as ours, we will better understand the potential to improve health outcomes and reduce costs in this setting. Additional long term studies are an essential prerequisite to help prove the case for routine iron repletion in such patients. Further work to determine the pathophysiology of ID in HFrEF as well as the precise mechanisms whereby I.V iron produces health benefits is required to optimize treatment modalities in the context of integrated HFrEF management.
Limitation
We acknowledge a number of limitations that highlight a need for further studies using appropriate powering and trial design. First, our study has a small sample size and was therefore not high powered (70%) to permit statistically robust conclusions or claims for the mortality benefit. Second, this is a single center study in a unit with a great deal of experience of managing HFrEF patients using IV iron as part of the standard of care. Third, we investigated the effects of IV iron in patients with ID and anaemia, and we cannot extrapolate the observed long term improvements to patients with HFrEF and ID who have not yet developed anaemia.
Conclusion
Although with a small number of patients, this is the first long term-term study IV iron treatment in patient with HFrEF, ID anaemia and CKD. We would suggest that an initial repletion with approximately 1000 mg IV iron is beneficial in symptomatic patients and that further IV iron doses of approximately 200 to 500 mg iron every 1 to 2 years thereafter may confer multiple benefits to HFrEF patients and to healthcare systems. The demonstration of increased survival, delayed and reduced hospitalizations, and improved cardiac function and HRQoL supports the growing body of evidence that IV iron might be considered an essential, well tolerated component of HFrEF treatment where ID is identified. Suitably-powered, randomized clinical trials are required to confirm the benefits demonstrated in our trial to support the use of IV iron in symptomatic HFrEF patients with confirmed ID.
* Data are reported as mean ± SD
* including the 1000 mg administered per original protocol; no iron administered between months 7 and 12 in the first year.
**p<0.01 vs control at same time point