COPD is a condition characterized by partially reversible airflow limitation that is associated with an increased inflammatory response to noxious particle [1]. Cigarette smoking is a primary causal factor of COPD but chronic infection and other environmental exposures such as coal mining dust, diesel exhaust particle are also associated with COPD [2,3]. Cigarette smoked exposure and toxic gases initiates the airway inflammatory cascade, which includes various type of inflammatory cells such as macrophages, neutrophils, T and B lymphocytes and epithelial cells as well as increased concentrations of IL-8 [4].
IL-8 is a chemokine produced by macrophages and epithelial cells. IL-8 belongs to α-chemokine family and it acts as primarily one of the most potent neutrophils chemoattractants. IL-8 may be regarded as proinflammatory and epithelial derived mediators in COPD [5,6]. In contrast to IL-8 several other chemotactic factors for neutrophils have been reported and they differ in chemical structure, receptor specificity and origin. These comprise of platelets activating factor and leukotrienes B4 [7,8]. Among these factors IL-8 is more important because it is resistant to many denaturing treatments, resembling oxidation or hydrolysis and it persist in its active form in tissue for a long time [9,10]. In case of COPD patients, inflammation may be illustrated by an increased number of neutrophils count and elevated level of IL-8 in the sputum [11]. The increased levels of IL-8 are also associated with diminished Forced Expiratory Volume in one second (FEV1) and increased dyspnea and grading as Global initiative for Chronic Obstructive Lung Disease (GOLD) in COPD patients which supports the relationship of IL-8 inflammatory mediators with COPD [12,13,14]. These findings indicate the significance of IL-8 inflammatory marker as a new mechanism for the identification of COPD in future.
The aim of the present study was to investigate whether the expression of IL-8 is altered in COPD patients when compared to healthy controls, whether feasible alterations are present previously in circulation or if feasible alterations occur when it enters the airways. Therefore, inflammatory cell expressions of IL-8 were assessed in peripheral blood (systemic compartment) and induced sputum (airway compartment) from COPD participants and healthy controls. In addition, we established the relation of smoking status to IL-8 and spirometric parameter.
Materials and Methods
This case control study was conducted from March 2014 to November 2016 at the department of respiratory medicine, King George’s Medical University (KGMU), Lucknow, India in accordance with the Declaration of Helsinki and approved by Ethics committee of KGMU. The sample size was calculated on the basis of difference of mean IL-8 levels in COPD and healthy control group [15,16]. As per calculated sample size, a total of 81 subject with COPD and 81 healthy controls with age of 40 years or older were recruited in this study. Consecutive diagnosed cases of COPD were enrolled from the department of respiratory medicine KGMU. The diagnosis criteria of COPD patients were based on Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria [1]. The COPD patients had cough or sputum for two weeks before the recruitment as well as Forced Expiratory Volume in one second/Forced Vital Capacity ratio (FEV1/FVC) < 70% of predicted value and FEV1 < 80% of predicted value after salbutamol administration.
The age matched male and female volunteers were recruited as healthy controls from the general population. These control subject had no specific respiratory disease, not receiving any medication specifically inhaled bronchodilator or corticosteroids, FEV1 > 80% and FEV1/FVC > 70% predicted value.
Subjects were excluded if they had a pregnancy, diabetes, hypertension and any other associated diseases. All the participants in this study gave written informed consent. Demographic characteristics and medical history with smoking status of all the participants were recorded. Blood and sputum samples were obtained from all studied participants.
Sputum Induction
Induced sputum sample was obtained from the subjects according to guideline of European Respiratory Society’s Task Force, as previously described [17,18]. Subject inhaled 200μg of salbutamol followed by inhalation of 3% - 5% hypertonic saline for three times with five minutes duration by an ultrasonic nebulizer. The sputum samples were collected into sterile pots.
Sputum Processing
The collected sputum samples were processed within two hours of sputum induction. Sputum was separated from saliva and transferred into centrifugal tube, mixed with an equal volume of freshly prepared 0.1% Dithiotreitol solution (DTT; Sputolysin; Sigma Aldrich Co., USA) and incubated in a thermostat at 37°C for 20 minutes. To, minimize the effect of DTT on cell suspension; DTT was diluted with equal volume of PBS. The mixture was filtered through a 48 μm gauze and centrifuge at 1000 rpm for five minutes. The cell- free supernatant was collected and stored at -80°C for further analysis.
Peripheral Blood Neutrophils Isolation and Culture
Peripheral blood neutrophils were isolated according to Clark RA and Nauseef WM methodology as described previously [19]. Neutrophil cells were resuspended in RPMI 1640 (Gibco, Invitrogen, USA) with 1% fetal calf serum, 10mM HEPES (4-(2- hydroxyethyl)-1-piperazineethanesulfonic acid) and 1% antibiotics (Penicillin/ Streptomycin). The cells were cultured on a concentration 1×106 cells/mL with LPS (100 ng/mL, Escherichia coli, sigma USA) stimulation in 5% CO2 at 37°C for 24 hours. Cell free supernatant was prepared for ELISA test and cell pellets stored in RLT buffer (Qiagen, Germany) at -80°C for RNA extraction.
RNA Extraction and cDNA Preparation
Total RNA was extracted from neutrophil cell pellets by using a commercially available RNA extraction kit (Pure linkTM RNA Mini Kit, Ambion, USA) according to the manufacturer instructions. After the isolation of RNA, DNAase I treatment was given to assure the purity of RNA without DNA contamination. The quality and quantity of extracted RNA was determined by UV absorbance at 260/280 nm. High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA) was used to synthesize cDNA from 100 ng of RNA and it was stored at -80°C for further experiments.
Real Time PCR Analysis
Quantitative real time PCR for gene expression of IL-8 was performed using SYBR Green Chemistry (Applied Biosystem, USA) with gene specific primer [20], by ABI 7500 Real Time PCR Machine (Applied Biosystem, USA). The results were calculated using 2-ΔCt relative to the house keeping gene (β-actin) as an internal calibrator.
Measurement of IL-8 Level
IL-8 protein levels were estimated in cell culture supernatants of peripheral blood neutrophils and sputum supernatant sample of COPD patients along with healthy controls. The estimation of IL-8 protein was performed by commercially available ELISA kit (R & D Systems, Minneapolis, MN, USA) following manufacture protocol.
Statistical Analysis
Data were analyzed using Graph Pad Prism version 5 (Graph Pad software Inc.; La, Jolla, CA, USA). All data were expressed as median with interquartile range unless otherwise indicated. Chi-square test used for categorical data analysis and group were compared by unpaired t-test. The comparison of groups was performed by non-parametric Kruskal–Wallis test followed by Dunn’s post-hoc test. Spearman’s rank correlation test was used for association between data. Results were considered significant when p < 0.05.
Results
The clinical characteristics of COPD patients and healthy control are summarized in [Table/Fig-1].
Clinical characteristics of COPD and healthy control participants.
| Number | Healthy controls | COPD | p-value |
|---|
| 81 | 81 |
|---|
| Age Years, mean±SD | 52.97±11.70 | 55.65±9.97 | 0.11a |
| Gender M/F | 54/27 | 70/11 | 0.003b |
| Height in cms, mean±SD | 160.90± 9.62 | 160.29±10.12 | 0.69a |
| Weight in kgs, mean±SD | 63.58±13.52 | 50.61±10.38 | 0.001a |
| BMI kg/m2, mean±SD | 24.51±4.45 | 19.77±4.21 | 0.001a |
| Smoking, Never/Smoker | 55/26 | 9/72 | 0.001b |
| Pack years, mean±SD | 19.27±4.41 | 28.48±14.65 | 0.001a |
| Post FVC %, mean±SD | 89.48±10.34 | 60.55±13.73 | 0.001a |
| Post FEV1 %, predicted mean±SD | 89.90±7.84 | 38.44±10.10 | 0.001a |
| Post FEV1/FVC (%), mean±SD | 100.97±7.90 | 61.30±7.90 | 0.001a |
COPD: chronic obstructive pulmonary disease; FEV1: forced expiratory volume in 1 second; FVC: forced vital capacity; SD: standard deviation; Unpaired t-testa, Chi-square testb, Results are shown Mean± SD (Standard deviation) Where, p<0.05: Significant.
The subject groups were generally similar with respect to age. The COPD patients have greater smoking history and pack years as compared to healthy control and it was significant (p<0.001). There was a statistically significant difference between COPD and healthy control participant with respect to FEV1 %, FVC% and FEV1 /FVC % predicted. COPD patients and other participants did not report any adverse events throughout the study period. However, no significant changes in FEV1 before and after inhalation of hypertonic saline solution occurred.
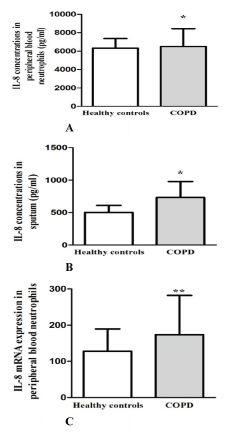
The level of IL-8, assessed by ELISA was significantly higher in peripheral blood neutrophils of COPD patients compared to healthy control (p=0.018) [Table/Fig-2A]. Participants with COPD also had a significantly higher level of IL-8 (p=0.011) [Table/Fig-2B] in sputum sample. COPD patients showed significantly increased the level of IL-8 mRNA expression in peripheral blood neutrophils (p=0.001)[Table/Fig-2C].
IL-8 level were assayed by enzyme linked immunosorbent assay and COPD patients showed significantly higher level of IL-8 in peripheral blood neutrophils (A) and sputum supernatant (B) compared with healthy control. IL-8 mRNA expression level in peripheral blood neutrophils from COPD patients and healthy control group (C). Data are presented as median value with interquartile range. *p<0.05 and **p<0.001 compared with healthy controls.
Impact of Smoking
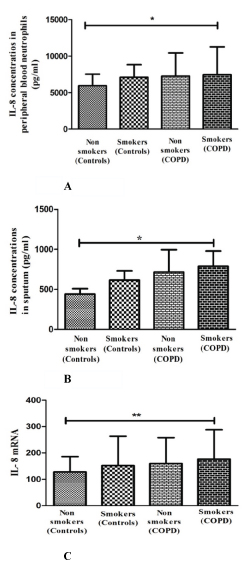
The impact of smoking was investigated by further analysis of IL-8 marker in smoker and non-smoker sub-groups of COPD patients and healthy controls. Smokers with COPD participants had significantly higher level of IL-8 in peripheral blood neutrophils (p=0.039) [Table/Fig-3A] and sputum supernatant (p=0.026) [Table/Fig-3B]; when compared to smokers without COPD and non-smokers. The IL-8 mRNA level was also significantly increased in smokers with COPD participant (p=0.008) [Table/Fig-3C]. However, a positive correlation between pack year smoked with the levels of IL-8 in peripheral blood neutrophils (r= 0.42; p= 0.009) and sputum (r= 0.48; p= 0.002) and negative correlation with FEV1 % predicted (r= -0.37; p= 0.044) and FEV1 /FVC (r= - 0.47; p= 0.007) were observed in COPD patients.
IL-8 levels in peripheral blood neutrophils (A) and sputum supernatant (B) from smoker and non-smoker groups of COPD patients and controls. IL-8 mRNA expression level in peripheral blood neutrophils (C). Data are presented as median value with interquartile range.*p<0.05 and **p<0.001 compared with non smoking controls.
Discussion
This study investigated the response of IL-8 in both the systemic and airway compartments. The chronic persistent inflammation in COPD patients is directed by neutrophils which are the key effectors cells that can also mediate the systemic effects of chronic inflammation [21,22]. Investigation of IL-8 protein and mRNA in peripheral blood neutrophils of COPD patients could therefore provide a new approach into the molecular mechanisms modulating chronic systemic inflammation. In this study the level of IL-8 protein and mRNA expressions were examined in peripheral blood neutrophils in response to LPS stimulation. The level of IL-8 protein and mRNA was increased in peripheral blood neutrophils with LPS stimulation of COPD patients, demonstrating the potential for activation of chronic inflammation.
Noguera A et al., demonstrated that enhanced activation of systemic neutrophils in COPD patients, defined a potentiation of migratory and cytotoxic responses as well as up regulation of inflammatory genes [21,23-25]. Neutrophils may release the increased amount of IL-8 which adds to positive feedback circle in COPD. Baines KJ et al. reported that the level of IL-8 protein and mRNA, both was enhanced in peripheral blood neutrophils of COPD patients [11]. Oudijk EJD et al., found higher level of IL-8 in peripheral blood neutrophils in moderate and severe to very severe COPD compared to healthy controls [21]. Kawayama T et al., also showed the level of IL-8 higher in COPD from peripheral blood mononuclear cells (PBMCs) stimulated by LPS [26]. Similarly, another study on PBMCs showed increased level of IL-8 mRNA in COPD patients [27]. The current study also reports similar result indicating higher level of IL-8 protein and mRNA in peripheral blood neutrophils of COPD patients. These findings are suggestive of a significant role of IL-8 in systemic inflammatory process in COPD.
The induced sputum method is a non-invasive and reliable method sufficient in representing inflammation in airways such as that occurring in COPD as shown in previous studies [28,29]. Ishikawa N et al., found the higher level of IL-8 in induced sputum of COPD patients [30]. Keating VM et al., reported higher IL-8 level is in induced sputum of COPD while comparing it with asthmatic subjects [31]. In another study, Yamamoto C et al., also showed increased levels of IL-8 in the sputum of COPD patients than asthmatic patients and normal controls [32]. Similarly, we also found the increased level of IL-8 in COPD patients from sputum sample, which is an agreement with previous study [30-32].
Cigarette smoking is well accepted as the major risk factor for COPD and our study also reports the greater history of smoking in COPD patients than control group. Cigarette smoke interacts with host factors which may produces inflammation and decline in lung function resulting in COPD [1]. There are several studies investigating the relationship between cigarette smoke and airway inflammation. Mio T et al., study in bronchial epithelial cells isolated from major bronchi showed response to tobacco smoke by releasing IL-8 [33]. Takizawa H et al., found increased levels of IL-8 mRNA and IL-8 protein release in small airway epithelial cells in smokers [20]. The increased level of IL-8 in induced sputum from COPD patients compared with smoking control groups were also reported by Wang S et al., [34]. The present study demonstrates that the level of IL-8 protein and mRNA was significantly increased in smokers with COPD than healthy smokers and non-smoker control subjects.
There are several studies investigating the correlation of IL-8 level with neutrophils and spirometric data. Keating VM et al., and Yamamoto C et al., reported a positive correlation between IL-8 level and neutrophil numbers [31,32]. This could not be demonstrated in our study for the reason that characterization of cellular components of the sputum was not performed. Yamamoto C et al., demonstrates a negative correlation between the IL-8 levels in induced sputum and FEV1/FVC ratio in COPD patients [32]. Another study reported serum IL-8 level positively correlated with annual rate of fall in FEV1 ratio [35]. However, we could not establish any relationship between IL-8 levels and spirometric parameters in current study. This may be due to methodological variation in above mentioned studies and limited numbers of subjects in study.
Previous studies have demonstrated the relationship of smoke pack year with IL-8 levels. Takizawa H et al., found that IL-8 mRNA level was significantly correlated with smoke pack year [20]. Kanazawa H et al., demonstrate a correlation between serum IL-8 level and pack year smoke in emphysema patients [35]. The present study, correlation analysis showed that there was a positive correlation between pack year smoked and IL-8 level in blood and sputum of COPD patients. Moreover, pack year smoked is negatively correlated with spirometric parameters. These findings suggest that smoke load may be an indicator to predict the smokers that will develop COPD in future. This exposure thinks to be the preliminary stage of COPD pathogenesis. Since, we have not performed long term follow up study therefore, we could not set any clinical significant cut off value for IL-8 level and pack year smoked which may be helpful to predict the healthy smokers at risk. However, we think our current finding provides notable support for investigators in further studies and they include long term follow up in their methodology.
Limitation
The current study has certain limitations which should be emphasized. First, being the numbers of COPD patients and healthy smokers were small. Second, we could not establish the relationship between IL-8 levels and inflammatory cells as cellular components analysis of the sputum was not performed.
Conclusion
This is the first study to investigate the level of IL-8 both in systemic and airway compartment of COPD in north Indian subjects compared with healthy controls. The increased level of IL-8 in peripheral blood neutrophils and sputum provides further evidence that COPD is a multicomponent disease, involving both airway and systemic inflammation. The correlation of pack year smoke to IL-8 level might help in further studies to explore the relationship between cigarette smoking load and inflammatory response.
COPD: chronic obstructive pulmonary disease; FEV1: forced expiratory volume in 1 second; FVC: forced vital capacity; SD: standard deviation; Unpaired t-testa, Chi-square testb, Results are shown Mean± SD (Standard deviation) Where, p<0.05: Significant.