Dengue Virus (DV), a Flavivirus and Chikungunya Virus (CHIKV), an Alphavirus belong to the families Flaviviridae and Togaviridae respectively. DV and CHIKV are transmitted by the same species of mosquito, Aedes aegypti and also by Aedes albopictus and both the viruses are known to cause acute febrile illness with almost identical symptoms in the early phase of infection [1]. DV has four serotypes referred to as DV-1, DV-2, DV-3 and DV-4. Full spectrum of clinical illness ranging from an asymptomatic or mild febrile illness to severe illness i.e., Dengue Haemorrhagic Fever (DHF) and Dengue Shock Syndrome (DSS) has been reported by all the four serotypes [2]. CHIKV causes acute febrile polyarthralgia, arthritis and a distinctive rash. Complications of chikungunya include myocarditis, hepatitis and also ocular and neurological disorders [3]. As CHIKV has clinical similarity to DV, it can be wrongly diagnosed as dengue infection, mainly in the regions of dengue endemicity. Asia, Africa and North America reported the epidemics of dengue in the 1780s, while Tanzania reported chikungunya in 1952 [4,5]. In India, major epidemics of dengue and chikungunya were experienced in Kolkata during 1963-1964. [6,7]. At present, 50–100 million cases are reported every year and 40% of global population is at risk of dengue infection [8]. Epidemics of chikungunya were recorded in Africa and Asia through the years of 1960s and 1990s [9]. CHIKV has shown resurgence in India since late 2005, affecting 1.56 million people, and has spread to more than 17 states/Union Territories, and is showing footprints in other areas too. Many of these areas are dengue-endemic settings [10]. Recently, a rise in number of dengue and chikungunya cases has been reported from Delhi and various other states of northern India [11].
An emergence and regular occurrence of dengue positive cases in Jammu has been observed since 2011 [12]. Although chikungunya infection had not been reported from Jammu, but in view of epidemic of chikungunya in the northern states of India including the neighbouring regions of Punjab, a need for diagnosis and timely management was felt [11]. The present study was planned to observe the pattern and status of these two arboviral diseases in this region in the calendar year 2016. Due to the similarity in the signs and symptoms, specific laboratory testing is required for diagnosis and management. Also currently the emergence of Zika Virus epidemic in some parts of the world with a common vector and similar clinical presentation necessitated an Active Surveillance for Zika Virus in Jammu region under National guidelines [13].
Materials and Methods
The present prospective, cross-sectional study was conducted in a tertiary care teaching hospital, Jammu and due permission was taken from the Institutional Ethical Committee (IEC). Jammu Province has a total of ten districts, each having a district hospital at district headquater. The blood samples received from all these district hospitals as well as from outpatient departments and wards of Govt. Medical College, and associated hospitals, Jammu, a tertiary care teaching hospital were analysed in the Department of Microbiology, GMC, Jammu. A clinical case included a patient with fever and symptoms suggestive of dengue as per WHO guidelines [14]. There were no exclusion criteria for processing of the collected samples. About 2 ml to 3 ml of blood was collected from each patient using strict aseptic precautions. Serum was separated by centrifuging samples at 3000 rpm for five minutes and tested immediately. In case of delay in processing, sera were stored at a temperature of 2°C-8°C. Depending upon the duration of illness, a total of 808 sera were collected and serologically tested, for demonstration of NS1 antigen (fever =/<5 days) or anti DV IgM antibodies (fever >5 days). NS1 antigen detection was done using DENV Detect NS1 ELISA kit, by In Bios International Inc. USA and IgM anti dengue antibodies were detected by NIV dengue IgM capture kits by NIV, Pune, India. The procedures were performed as per the instructions provided by the respective kits and are as follows:
a) DENV DetectTM NS1 ELISA: A 50 μl of undiluted serum was added directly to each antibody coated well containing 50 μl sample diluent for each sample. The assay plate was incubated at 37°C for one hour and then washed. To each well, 100 μl of conjugate solution was added and it was then incubated for 30 minutes. The microwells were washed thereafter and 100 μl of TetraMethyl-Benzidine/Hydrogen peroxide (TMB/H 202) substrate solution was added to these well. After 20 minutes of incubation at room temperature, 50 μl stop solution was added to each well and the colour density of the residue i.e., Optical Density (OD) was read within one minute at the wavelength of 450 nm. The cut-off value was calculated based on the average OD value obtained from control sample.
b) NIV dengue IgM Capture ELISA: To the microwells coated with anti human IgM antibodies, 50 μl of 1:100 diluted serum and controls were added to ELISA plate. The assay plate was incubated at 37°C for one hour and then washed. About 50 μl of DEN antigen conjugate solution complex was added to the assay plate and was incubated for one hour. Now, 50 μl of antidengue monoclonal antibody was added to each well, followed by incubation for 1 hour and all the wells were washed. A 50 μl of avidin-HRP was added to each well and again incubated for 30 minutes. A 100 μl TetraMethyl-Benzidine/Hydrogen peroxide (TMB/H202) substrate solution was added to each well, after washing and incubated for 10 minute at room temperature. The OD was read within 30 minute, of addition of 100 μl stop solution, at the wavelength of 450 nm. The cut-off value was calculated using the formula (Mean Absorbance Negative Control x 3.0). Patients with positive NS1 antigen and anti dengue IgM were considered positive cases for dengue viral infection.
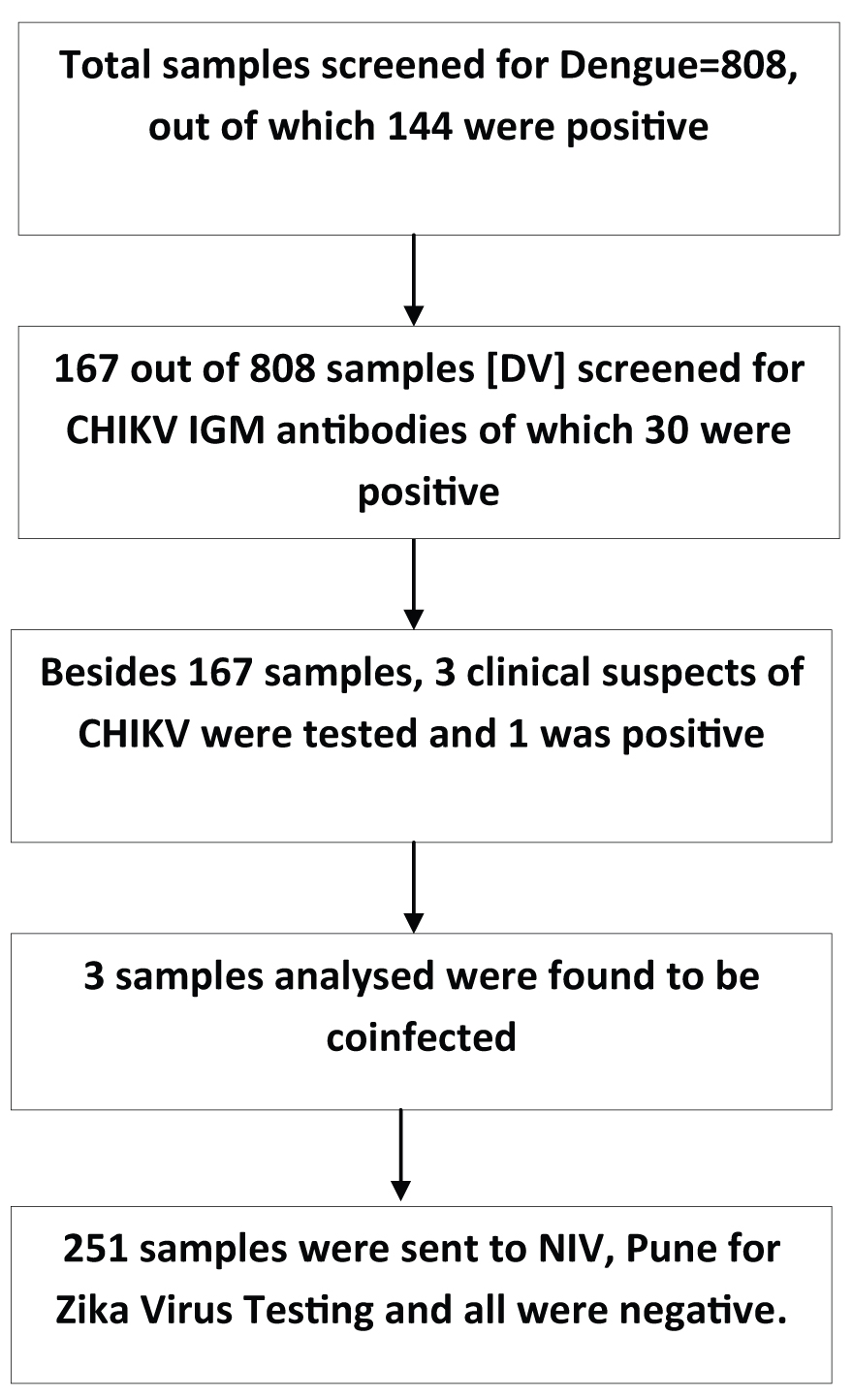
The clinical suspects with fever, arthralgia, myalgia with/without rash were included for serological diagnosis of chikungunya [11]. In the next step 170 samples were tested for CHIK anti IgM antibodies (in patients who had history of fever for >5 days). Besides 3 clinical suspects of chikungunya, only 167 dengue sera (7 positive and 160 negative) were tested, keeping in view the presence of Chik dengue coinfection and also the paucity of NIV CHIK IgM Capture ELISA kits provided by NIV Pune. Briefly, 100 μl of 1:100 diluted serum samples was added to the anti IgM coated wells, incubated at 37°C and washed. About 50 μl of CHIK antigen was added to each well followed by incubation and washing. In the next step 50 μl of Biotin-labelled Anti CHIK Monoclonal antibody was added, incubated and washed. This was followed by addition of 50μl of Avidin –HRP to the microwells. After incubation, the microwells were washed and 100 μl of TMB/H2O2 substrate solution was added to each well. After 10 minutes of incubation at room temperature, 100 μl stop solution was added to each well and the OD was read within 30 minute at the wavelength of 450 nm. The cut-off value was calculated using the formula (Mean Absorbance Negative Control x 3.0) as per kit literature. Finally, 251 samples (all stored available DV and CHIKV negative) were sent to NIV Pune for ZIKA virus testing as per National guidelines [13].
Statistical Analysis
The data was collected, tabulated and analysed. Pearson’s Chi-square test was used as test of significance. A value of p<0.05 was considered significant.
Results
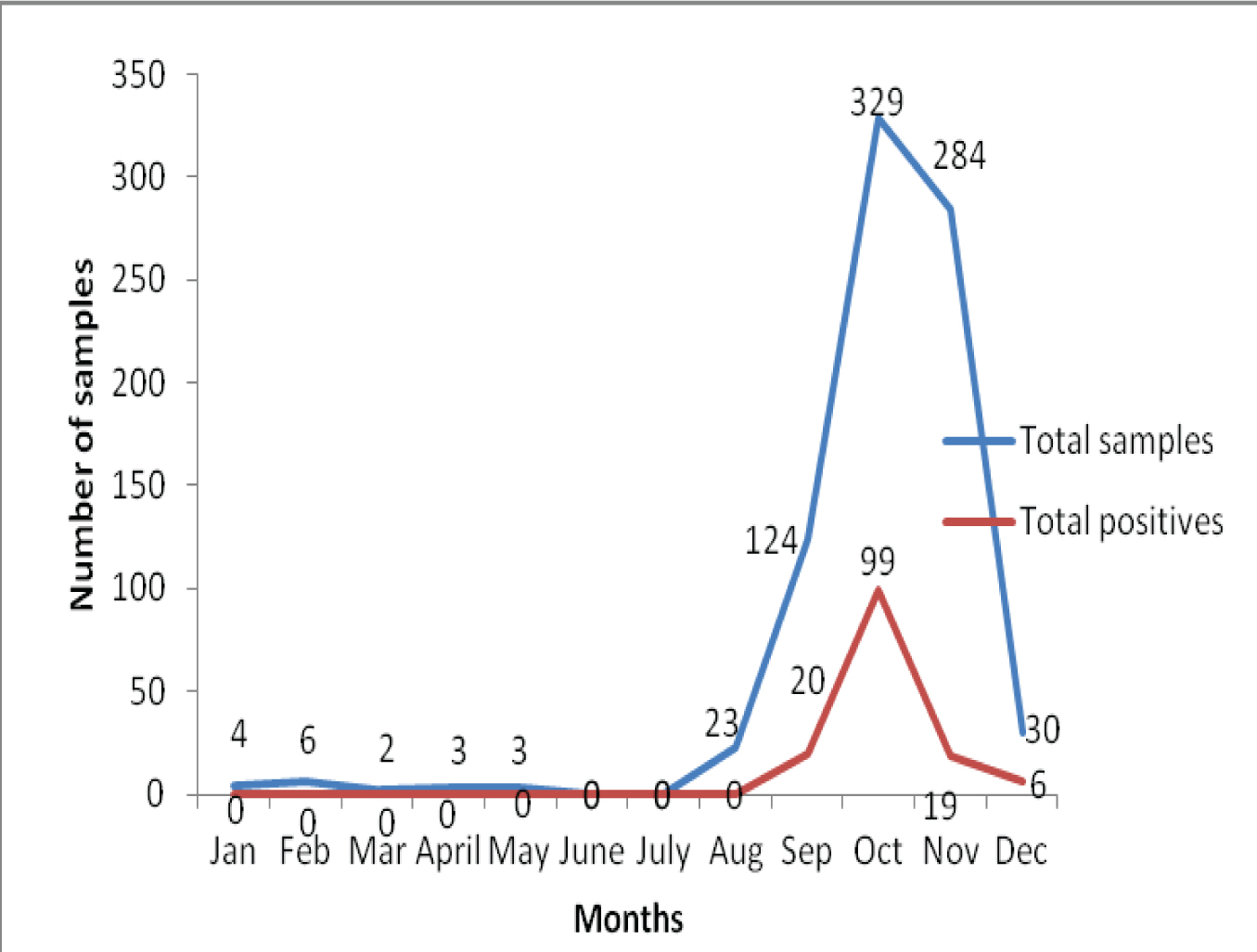
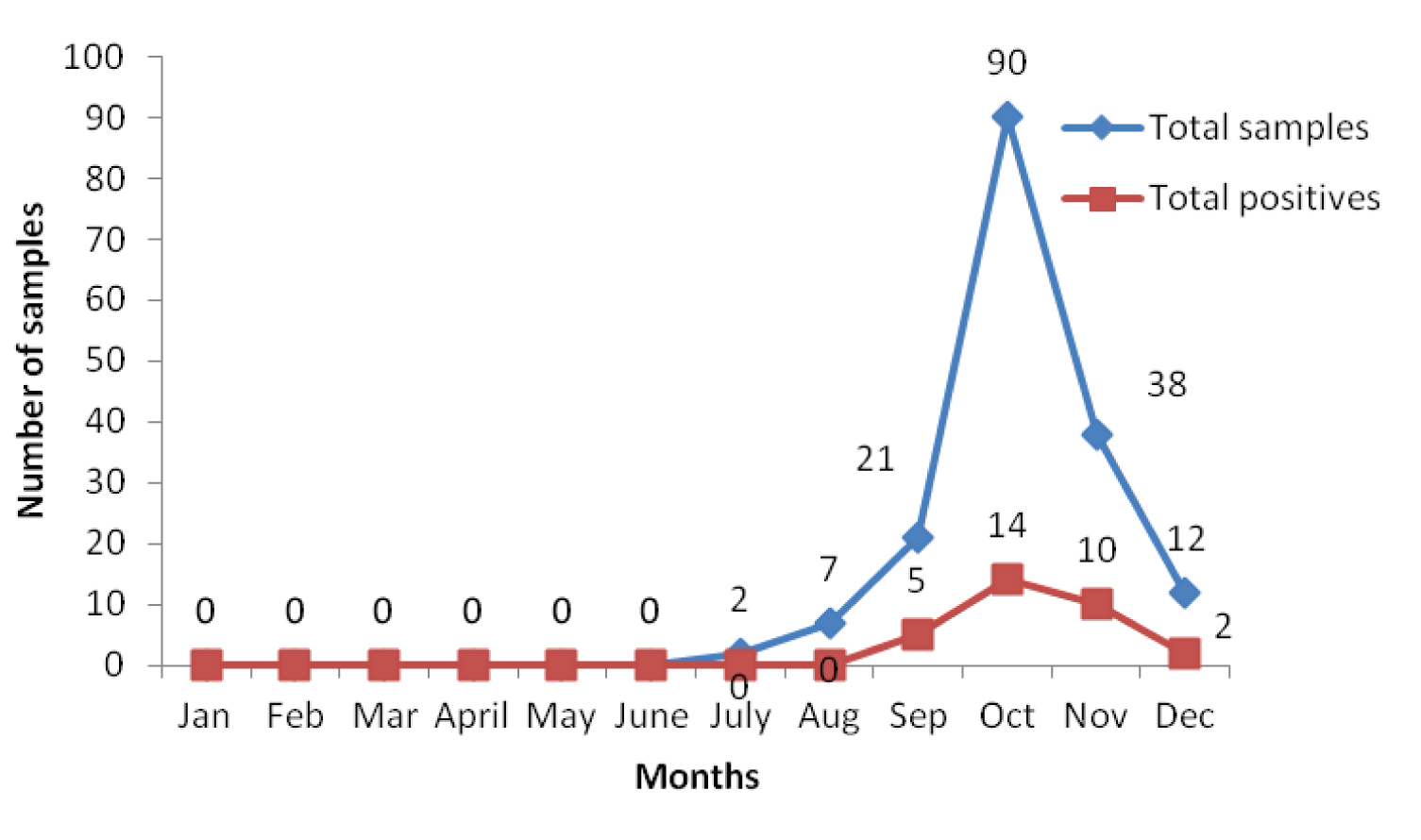
A 17.82% (144/808) of the total samples were positive for dengue, of which 21.2% (123/579) and 9.1% (21/229) were positive for NS1 Ag and anti dengue IgM Antibodies respectively. A 18.20% (31/170) were positive for antiCHIK IgM antibodies. Since 31 samples were positive for CHIKV, they were further analysed for co-infection of DV-CHIKV. The results showed that 3 out of 31 were coinfected. The DV-CHIKV coinfection positivity rate was 9.67% (3/31). The association of dengue on the basis of sex was found to be highly significant (p<0.001). Of the 144 dengue positive cases, 119(82.63%) seropositives were detected during the months of September and October collectively and 5, 14, 10 and 2 positive cases were observed for chikungunya during the months of September, October, November and December respectively in the year 2016 as shown in [Table/Fig-1] and [Table/Fig-2].
Monthly distribution of dengue positive cases (both NS1 and IgM).
Month-wise distribution of the serum samples tested for chikungunya.
Sex wise distribution of the serum samples for dengue and chikungunya infections is shown in the [Table/Fig-3]. The association between serum samples on the basis of sex distribution was found to be highly significant for dengue cases (p=0.0000) but no such association was found for chikungunya cases (p>0.05). The following flow chart explains the modus operandi followed in the study [Table/Fig-4].
Distribution of dengue and chikungunya cases on the basis of sex in the calendar year.
| Month | Sex wise distribution of Dengue | Sex wise distribution of Chikungunya |
|---|
| Male | Female | Total | Male | Female | Total |
|---|
| January | 2 | 2 | (0)4 | 0 | 0 | 0 |
| February | 4 | 2 | (0)6 | 0 | 0 | 0 |
| March | 2 | 0 | (0)2 | 0 | 0 | 0 |
| April | 2 | 1 | (0)3 | 0 | 0 | 0 |
| May | 3 | 0 | (0)3 | 0 | 0 | 0 |
| June | 0 | 0 | 0 | 0 | 0 | 0 |
| July | 0 | 0 | 0 | 0(2) | 0(0) | 0(2) |
| August | (0)15 | (0)8 | (0)23 | 0(5) | 0(2) | 0(7) |
| September | (16)9 | (4)28 | (20)124 | 4(16) | 1(5) | 5(21) |
| October | (80)2 | (19)3 | (99)329 | 8(64) | 6(26) | 14(90) |
| November | (13)2 | (6)20 | (19)284 | 7(30) | 3(8) | 10(38) |
| December | (4)20 | (2)10 | (6)30 | 2(9) | 0(3) | 2(12) |
| Total | (113) | (31)1 | (144)808 | 21(12) | 10(44) | 31(170) |
A) Chi-square (x2) = 6.7688, Df = 1, p = 0.009276
B) Chi-square (x2) = 0.8034, Df = 1, p = 0.360081
Flow chart depicting the results of the study.
Discussion
Dengue and chikungunya are the most important emerging and re-emerging arboviral infections of the tropical and subtropical regions. A regular occurrence with periodic upsurges has been noted for dengue infection in most parts of India and in the last decade a progressive spread of chikungunya from south to north Indian regions has been noticed [15,16]. From the Jammu province, the Sero-epidemiological trends of dengue for five years i.e., 2011–2015 have already been reported [12]. Being the first year of emergence of chikungunya and also dengue-chikungunya co-infection, the present baseline study is the first original report to form a basis to initiate management of patients and plan and execute effective regional arboviral control measures and to further study the Molecular Characteristics of the locally prevalent dengue and chikungunya viruses. Also a sero-surveillance of another impending Aedes Borne Viral epidemic with ZIKA virus has been initiated under National guidelines. Chikungunya re-emergence has been associated to various reasons such as globalization, inadequate mosquito control, waning herd immunity and even due to E1 gene mutation A226V leading to a consequential rise in CHIKV infectivity for Aedes albopictus also [17].
In the current study, 17.82% samples were serologically positive for dengue infection which is in line of agreement with the results reported by a study from Kanpur [18]. However in contrast, Ekta et al., reported a higher rate of 44.56% of seropositivity in their study [19]. Other studies from Delhi and Gwalior reported much higher seropositivity rates to the tune of 95% and 65% respectively [20,21]. Two factors can be attributed to higher seropositivity reported in other studies i.e. i) heavy load of migratory floating population from dengue endemic states of India and ii) increase in rate of transmission due to exorbitant dengue virus activity. One more factor could be inadequate water storage which may act as breeding grounds for the mosquitoes. A lower dengue seropositivity (17.82 %) in 2016 compared to a higher seropositivity in previous years [12] may be due to development of herd immunity amongst the local population. According to National Vector Borne Disease Control Programme (NVBDCP), around 58,136 cases of Chikungunya have been reported across the country in the year 2016 [11]. Aedes aegypti, mainly and Aedes albopictus to a lesser extent are the vectors of transmission of dengue and chikungunya. Flight range of these mosquitoes is less making the outbreaks to occur in clusters, especially in congested localities. Due to high viraemia in chikungunya, infected mosquitoes tend to transfer the disease to more than one person as residual blood in the proboscis carries the virus in infecting dose [11].
However, regular dengue outbreaks have occurred in the Jammu province but chikungunya outbreaks have never been reported, despite the fact that the climate of Jammu is favourable for growth and spread of virus. Human migration patterns disperse both vectors and viruses in new areas. Initial epidemics of dengue, more so in North India, have contributed to heightened awareness among the medical fraternity and also an improved access to quality diagnostics has led to better detection [22].
In our study 3/31 (9.67%), CHIKV and DV coinfection cases have been reported. The coinfection significantly increases the morbidity and mortality, especially in the immunocompromised, debilitated and old patients. Co-infection is not very unusual as both arboviral diseases are transmitted by the same Aedes mosquitoes. Evidence for CHIKV and DV co-infection has been reported in 13 out of 98 countries by Furuya-Kanamori L et al., where both CHIKV and DV epidemic/endemic transmission has been reported [23].
A monthly distribution of the cases of dengue and chikungunya infections in this study shows an increase from the month of September onwards which concurs with other outbreaks in India [19]. Seasonal variation was substantiated by gradual increase in cases from August and it peaked in October.
The correlation between occurrence of both arboviral infections and monsoon season is clearly evident in this study and was equally supported by the authors in their previous studies [9]. Seasonal variation reflects the need for local health authorities to execute effective prevention and control measures.
Sex wise analysis revealed a male predominance, which is consistent with observations in other Indian studies [24]. Males are usually outdoor during the day time for work or related activities, hence are more vulnerable. Although no sample was found to be positive for Zika virus, recent report by WHO confirming three positive cases from India should not make us complacent [25].
Limitation
The main limitation of the current study was that all the serum samples were collected from the government hospitals/institutions. So, the patients going to private sector could not be included in the study; hence, there is an expected under reporting.
The serotyping for all the four serotypes was not done and cases for DHF and DSS were not looked for.
Conclusion
The present study has revealed that dengue has become a constant seasonal alarm in this region of India. Chikungunya and its coinfection with DV are also showing its footprints and health planners need to be aware of importance of its early recognition and prompt diagnosis. Continuous surveillance and rapid implementation of mosquito control measures is the need of the hour. Zika virus is another looming threat on the horizon for public health planners in India.
A) Chi-square (x2) = 6.7688, Df = 1, p = 0.009276
B) Chi-square (x2) = 0.8034, Df = 1, p = 0.360081