Cancer has been the focus of most health planning policies due to high prevalence, morbidity and mortality associated with it. Today, survival rates and life expectancy has increased compared to the past, because much progress has been achieved in the treatment and prevention methods. There are different kinds of cancer diagnosed in women of which breast cancer is very common [7]. According to the World Health Organization, in 2015 the annual breast cancer deaths were about 571,000 people worldwide. This malignancy is the fifth leading cause of cancer death in the world [8].
Many studies have been conducted on the use of pomegranates for the treatment of infectious diseases such as bacterial, fungal and parasitic infestations [10,11], improvement of sperm quality and cancer with the development of environmental contaminants and use of chemical drugs [12-14]. Several studies suggest that different parts of pomegranate fruit have anti-proliferative, anti-angiogenic and anti-aromatase properties on human breast cancer cell lines and chemopreventive properties on breast cancer in mice [15-17]. The beneficial effects of pomegranate seed may be due to its polyphenol compounds [18]. Polyphenols found in fruits and vegetables have anti-inflammatory and anti-cancer activity in vitro and in vivo. Several reports indicated the cytotoxic activity of polyphenols in the pomegranate extracts may prevent and treat breast cancer and other cancers [17,19, 20].
There is high incidence of breast cancer in the world, including Iran, and for this reason the studies for finding new ways of prevention and treatment are very important. In some countries, there are several reasons that may cause rise in risk of breast cancer such as pollution, stress, diet and lifestyle of the individuals. Foods that have antioxidant activity play an important role in cancer prevention and reduction [21-23]. Despite other treatment modalities such as surgery, chemotherapy and radiotherapy, cancer mortality is still very high. Furthermore, the destructive effects of chemotherapy and radiation therapy on normal dividing cells, is the major disadvantage of this process in the treatment of cancer [24]. Given the above, in recent years, global attention to the natural products and dietary supplements which have anti-cancer properties has increased [25]. According to our previous study, PSO has a positive effect on the fertilization potency of rats [26]. This result may be due to the presence of several kinds of antioxidant compounds in this oil. These compounds can work like anticancer reagents and there are a few studies to support this [5,20]. In present study, the effect of the oil on two human breast cancer cell lines was investigated.
The main goal of this study was to assess the potential inhibitory effects of PSO on human breast cancer cell lines metastasis.
Materials and Methods
This experimental study was conducted at Cellular and Molecular Research Center of Yasuj University of Medical Sciences, Iran from March 2015 to March 2016. Ethics Committee approval was obtained from the Institutional Ethics Committee of Yasuj University of Medical Sciences, prior to the commencement of the study.
Preparation of Seed Extracts
Pomegranates were collected from various places of Arsanjan region of Fars Province, South of Iran. The seeds were dried and powdered; 30 gm of the resulting powder was mixed with 150 mL of hexane into a flask and put into the shaker for 24 hours at room temperature. Then the resulting solution was poured into the plates and incubated at 37°C for 24 hours. A 2000 μg resulting oil was resolved in a minimal amount of Dimethyl Sulfoxide (DMSO) and the solution was reached to the final volume of 1 mL with RPMI-1640 medium (Gibco), this was a stock of pomegranate seed oil solution for subsequent studies [18].
Cell Culture
The cell lines, MCF-7 and MDA-MB-468 have been procured from the Iran Pasteur Institute and cultured in RPMI-1640 medium containing 10% Fetal Bovine Serum (FBS), 2 mM L-glutamine Penn- Strep solution (Gibco) and incubated in 5% CO2 incubator at 37°C, replacement of the culture medium was performed every three days. When the confluence reached to 70-80 percent, cell passaging was done using 25% Trypsin-EDTA (EthyleneDiamineTetraacetic Acid) solution [27].
MTT Assay
The viability of cells was determined by MTT assay. This method is based on the succinate dehydrogenase enzyme activity in mitochondria of living cells that turns the yellow MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) solution into the insoluble purple formazan crystals, which can be then dissolved in DMSO and measured with ELISA plate reader. Cell counting was performed by treating cell lines with trypan blue colour. A mixture of 10 μL of cell suspension and 10 μL trypan blue colour was used to count cell lines by neobar lam and Olympus 30X or 10X microscope objective lens. Dead and live cells can be distinguished by blue and yellow cytoplasm respectively. Approximately (1×105) cells were counted by inverted microscope and then transferred to 96-well plate and incubated for 24 hour at 37°C, then treated with different concentration of pomegranate seed oil (100-1200 μg/mL) and the volume of the each well was 100 μL. As a negative control, medium containing 0.3% DMSO without extract was used. Micro plates containing cell extract for 24, 48 and 72 hour were incubated in the same conditions. 10 mL solution of MTT (5 mg/mL) was added to each well and was incubated for three hours at 37°C. A 100 mL DMSO was replaced with incubated MTT medium. Then the optical absorbance was measured at a wavelength of 570 nm with ELISA reader (BioTek Elx800). The effect of each concentration was evaluated in three replications. Viability percentage of cells that were affected by the different concentration of the extracts was calculated by dividing the absorbance of treated wells to the negative control absorbance multiplied by 100. The concentration of the extract which reduces the cell viability to half, was considered as IC50, the results were analysed by SPSS 19.0 software (Statistical Package for Social Sciences) and represented in the form of mean±standard error of mean [28].
Colony Assay
An 1 X 103 cells were seeded in each well of a 6-well plate and incubated for 24 hour at 37°C, then each well was treated with different concentration of the extract (50-200 μg/mL), one well was treated with 0.3% DMSO solution considered as a negative control, then MCF-7 cell line for 11 days and MDA-MB-468 cell line for nine days were incubated in a CO2 incubator. Then the cells were washed with Phosphate Buffer Saline (PBS) and fixed with a mixed solution of acetic acid and methanol in the ratio of 3:1 and incubated at 37°C for 10 minutes and stained with crystal violet. Colonies greater than 50 cells were counted under an Olympus microscope [29].
Wound Healing
Both cell lines (MDA-MB-468 and MCF-7) were seeded in a 6-well plate and were allowed to replicate and cover 70% of the bottom of each well. After the formation of a cell layer on the bottom of the plate, by using the blue sampler tip, the bottom of wells was scratched both horizontally and vertically. Then the plate was rinsed several times with PBS solution for extracting the isolated cells from the medium, and then all wells were treated with the extract and cells were allowed to migrate towards the gap. Cell migration into the scratched area was analysed with an inverted microscope [30].
Cell Morphology
To determine changes in cell morphology by fluorescence microscopy, initially, 150×103 cells from both cell lines were cast in 24-well plates and were incubated at 37°C in a CO2 incubator. When a single layer of cells was formed, all wells were treated with different concentrations of the extract and after 24 hour, Hoechst 33342 solution (10 μg/mL) was added and incubated at 37°C in the CO2 incubator for 7 minutes. Then, Pyridinium Iodide (PI) was added (2.5 μg/mL) and kept in the dark area for 15 minutes and 10 μL of the suspension was placed on a slide and analysed with a fluorescence microscope. Maximum absorbance of Hoechst and PI were in 460 nm and 575 nm, respectively [31]. At least four areas of the slides were counted and the percentage of cells in apoptosis and necrosis were calculated according to the following formulae:
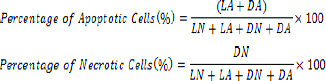
Where LN is living cells with normal cells and LA is living cells with apoptotic nuclei, DN is dead cells with normal nucleus and DA is dead cells with apoptotic nuclei.
Migration Assay
Breast cancer cells MCF-7 and MDA-MB-468 (5×105 cells/well) were incubated at 37°C in a 24-well plate for 24 hours, then 200 μL of the obtained suspensions with 100 μL of medium without Fetal Bovine Serum (FBS) was added to the top of the wells with 8 μm holes and 700 μL of medium containing FBS was added to the bottom of the wells. After 12-16 hours, the wells were washed with 100% methanol and buffer and stained with crystal violet stain and the number of cells that crossed the plate was counted by inverted microscope [32]. The experiment was performed three times.
Invasion Assay
The evaluation of invasion potential of the cells was done by using matrigel invasion chamber with 8 μm pores (Millipore, USA). This chamber is a hollow plastic chamber, sealed at one end with a porous membrane that was layered with an extra cellular matrix (matrigel). Cells were separated by trypsin from the culture flask and finally a suspension with the concentration of 5×105 cells/mL was prepared in culture medium without FBS. The cell suspension was added to the upper side of chambers and medium containing 10% FBS was added to the lower side of it. After 24 hours incubation at 37°C, cells that had not migrated were completely cleaned and the rest of them were fixed with the crystal violet and 100% methanol solution and counted using an inverted microscope. A total of 10 fields were counted per well [30].
Attachment Assay
Breast cancer cell lines MCF-7 and MDA-MB-468 were treated with different concentrations of PSO and incubated for 24 hours. Wells of 96-well plate was coated with laminin (10 μg/mL) and after an hour, rinsed with 0.5% and 1% Bovine Serum Albumin (BSA) solution, respectively. Then the cell suspension was added to the wells and incubated for 30 min. and the cells were fixed by para- formaldehyde and stained with crystal violet and adherent cells were counted by inverted microscope [33]. The experiment was performed three times.
Statistical Analysis
The statistical analysis of results were done by SPSS 19.0 software using One-way ANOVA followed by Post-hoc Tukey test and the level of significance was set at p<0.05.
Results
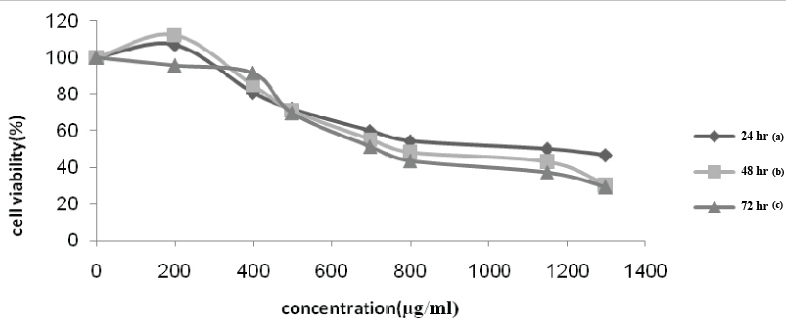
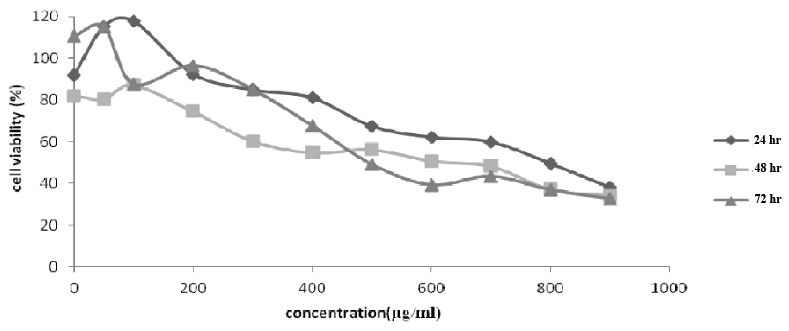
Cytotoxicity evaluation of pomegranate seed oil extract results using MTT assay after 24, 48 and 72 hour of treatment of both MCF-7 and MDA-MB-468 cell lines in the presence of various concentrations, respectively, are shown in [Table/Fig-1,2].
MCF-7 cell viability (%) vs. pomegranate seed oil concentration (μg/mL). Cell viability values obtained after incubation with various concentrations of the oil: (a) after 24 hour of incubation; (b) after 48 hour of incubation and (c) after 72 hour of incubation.
MDA-MB-468 cell viability (%) vs. pomegranate seed oil concentration (μg/mL). Cell viability values obtained after incubation with various concentrations of the oil: (a) after 24 hour of incubation; (b) after 48 hour of incubation and (c) after 72 hour of incubation.
Colony Formation
As the extract concentration for MCF-7 and MDA-MB-468 is increased, the colony forming cells/assay decreased [Table/Fig-3]. In experiments conducted at concentrations of 200 μg/mL for MCF-7 cell and at a concentration of 150 μg/mL for MDA-MB-468 cell lines, colony formation reduced significantly (p=0.003).
Colony assay for analyzing of cells aggregation with different concentration of extract treatment in MCF-7 & MDA-MB-468.
| Cell line | Dose: μg/mL | p-value* |
|---|
| 50 | 100 | 150 | 200 |
|---|
| MCF-7 Colony (Mean±SD) | 94.0±2.36 | 83.33±3.35 | 60±2.89 | 40±4.62 | 0.021 |
| MDA-MB-468 Colony (Mean±SD) | 71.67±1.76 | 58±1.73 | 32.67±2.33 | | 0.025 |
* p < 0.05 = Statistically Significant
Wound Healing Assay
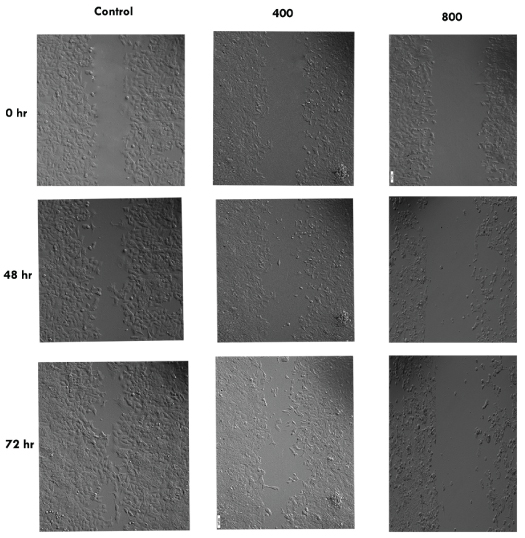
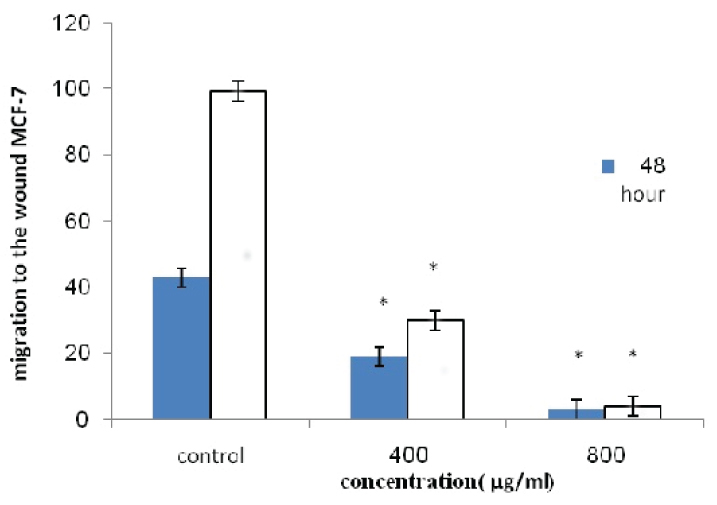
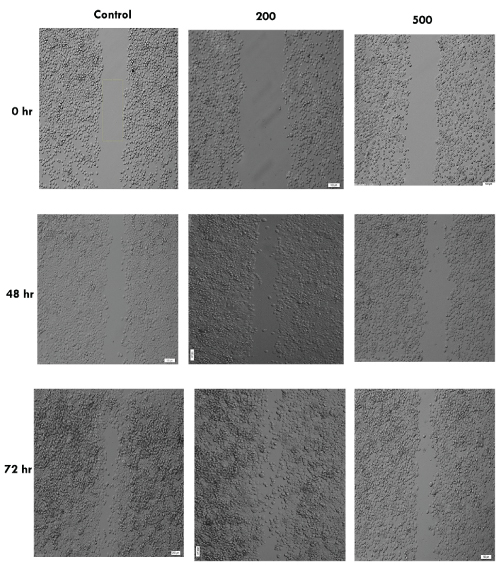
The cell migration plays an important role in pathological processes such as tumor formation and metastasis. For investigating the effects of pomegranate seed oil on cell migration, wound healing method was done. As shown in [Table/Fig-4,5,6 and 7], the cells treated with pomegranate seed oil compared with the control cells moved more slowly. In addition, unlike the treated cells, the cells in control-wells after 72 hours completely filled the gap.
Olympus IX71 inverted microscope of wound healing assay of MCF-7 cells treated with pomegranate seed oil at 0, 48 and 72 hr (100X) (data from three independent experiments; 400 and 800 μg/mL PSO).
The percentage of migration of MCF-7 cell line.
* indicates a significant difference at p<0.05 compared with control group.
Olympus IX71 inverted microscope of wound healing assay of MDA-MB-468 cells treated with pomegranate seed oil at 0 hr, 48 hr and 72 hr (100X) (data from three independent experiments.
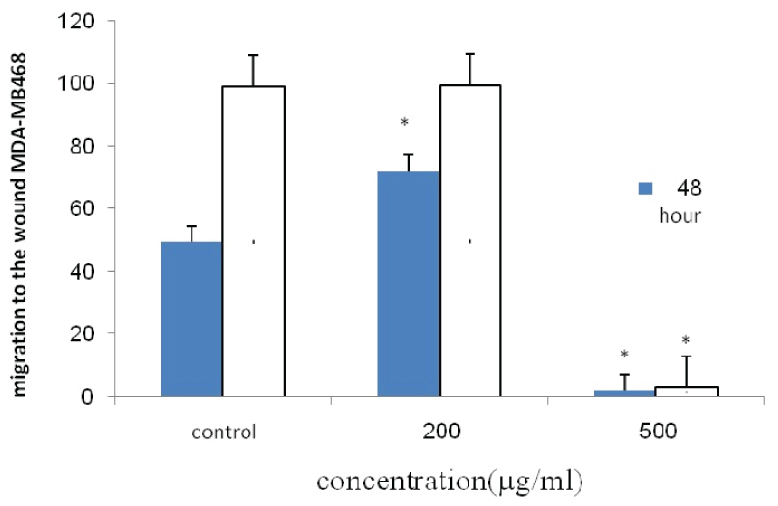
The percentage of migration of MDA-MB-468 cell line.
*Indicates a significant difference at p<0.05 compared with control group.
Cellular Morphology
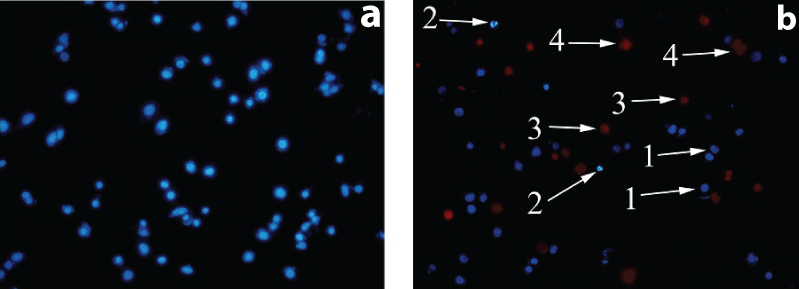
After treatment with pomegranate seed oil, it was shown that MCF-7 cell line in a concentration equivalent with 1000 μg/mL approximately 30.3% of cells were necrotic cells and 16.3% cells were apoptotic while it was found that MDA-MB-468 cell in a concentrations equivalent with 700 μg/mL, 35.7% was necrotic cells and 11% were apoptotic. The results showed that pomegranate seed oil is more likely to cause cell necrosis [Table/Fig-8].
Representative composite images show morphological changes of selected cancer cells (MCF-7, MDA-MB-468) detected with dual staining of Hoechst 33342/PI. Image A: normal cells. Image B: (1) viable cells with normal nuclei, (2) live cells with apoptotic nuclei, (3) dead cells with normal nuclei, and (4) dead cells with apoptotic nuclei.
Migration and Invasion Assay
Cytotoxic effect of pomegranate seed oil on two cell lines MCF-7, MDA-MB-468, respectively, at concentrations equivalent to 600, 1200 μg/mL and 400, 800 μg/mL indicate that the oil can prevent cell migration at the aforementioned concentrations [Table/Fig-9,10].
Different tests results in MCF-7 cell line treated by different concentrations of the extract.
| Assay | Dose: μg/mL | p-value* |
|---|
| 400 | 600 | 800 | 1000 | 1200 |
|---|
| Necrosis (Mean±SD) | 17.6±1.5 | | 21.3±1.2 | 30.3±1.6 | | 0.019 |
| Apoptosis (Mean±SD) | 7.3±1.2 | | 12.7±2.50 | 16.3±3.05 | | 0.021 |
| Migration (Mean±SD) | | 75±2.0 | | | 35.4±1.43 | 0.02 |
| Attachment (Mean±SD) | | 87.91±3.17 | 42.65±2.11 | | 2.96±1.2 | 0.0001 |
*p < 0.05 = Statistically Significant
Different tests results in MDA-MB-468 cell line treated by different concentrations of the extract.
| Assay | Dose: μg/mL | | p-value* |
|---|
| 200 | 400 | 500 | 600 | 700 | 800 |
|---|
| Necrosis (Mean±SD) | 12.3±3.21 | | 25.3±2.50 | | 35.7±2.52 | | 0.035 |
| Apoptosis (Mean±SD) | 4.3±1.52 | | 8.3±1.53 | | 11±2.0 | | 0.044 |
| Migration (Mean±SD) | | 78.02±1.43 | | | | 36.99±1.73 | 0.014 |
| Invasion (Mean±SD) | | 60.14±1.98 | | | | 34.79±1.36 | 0.023 |
| Attachment (Mean±SD) | | 94.73±1.08 | | 61.40±2.35 | | 5.44±1.2 | 0.048 |
* p < 0.05; t-Test.
Mcf-7 Cells Were Non-Invasive
Since the MCF-7 cells are non-invasive, these cells have not passed through matrigel. MDA-MB-468 cells are averagely invasive [Table/Fig-10], the cell invasion significantly compared to the control group is dramatically reduced at a concentration equivalent to 800 μg/mL.
Attachment Assay
Cell adhesion was studied using laminin, as shown in the [Table/Fig-2,3], in MCF-7and MDA-MB-468 cells, attachment has declined dramatically at the concentrations, 1200 μg/mL and 800 μg/mL, respectively.
Discussion
In the present study, the result of MTT assay showed that pomegranate seed oil can decrease the viability of two cell lines and other assays specifically wound healing, invasion and attachment assays showed that the oil may has anti-metastatic effect on invasive breast cancer cell lines; MDA-MB-468 and MCF-7.
Based on surveys conducted by Jing P et al., pomegranate seed contains the compounds such as phenolics, flavonoids, and pro-anthocyanidins [18]. The only unsaturated fatty acid is oleic acid in pomegranate seed oil and according to the expressed fruit combinations, this fruit has the antioxidant and anticancer effects [25]. The results of the study on two human breast cancer cell lines showed that the pomegranate seed oil can help in preventing the proliferation and migration of cancer cells. Another study by Jaramillo and his colleagues, showed that a diet containing flavonoids can be used as the preventive and therapeutic agents for various diseases including cancer [34].
In 2012, Zhang H et al., examined the effects of quercetin, and quercetin-5’, 8-disulfonate on breast cancer MCF-7 cell line and showed that these two compounds at concentrations equivalent to 100 μM at 24 and 48 hour caused inhibition of proliferation of cancer cells [35].
In the present study, the cytotoxic effect of PSO on two cancer cell lines MCF-7 and MDA-MB-468 were 1150 μg/mL and 842 μg/mL after 24 hour and 742 μg/mL and 700 μg/mL after the first 48 hour, respectively.
In 2009, Nagamine MK et al., studied the cytotoxic effects of butanolic extract from Pfaffia paniculata (Brazilian Ginseng) on MCF-7 cell line and found that the concentration of 400 μg/mL after 48 hours has the inhibitory effects [36] while in this study, the inhibitory effect of PSO on MCF-7 cell line was observed in a concentration equivalent to 742 μg/mL after 48 hr treatment concluding that butanolic extract is more effective than PSO.
The effect of PSO on several prostate cancer cell lines was investigated by Albrecht et al., and the results showed that this oil can inhibit the proliferation and invasion of cancer cells [37]. The results of the present study are in concordance with the previous studies.
The study by Kim et al., showed that pomegranate seed oil had an inhibitory effect on MCF-7 breast cancer cell and also prevents the invasion of cells in the matrix gelatin [38]. Present study showed that pomegranate seed oil has inhibitory effect on invasion of MDA-MB-468 but MCF-7 cell line is a non-invasive cell and the effect of this oil on MCF-7 cell line is on its viability.
Significant reduction has been observed in the multiplicity of carcinoma in colon of mice fed with pomegranate oil [39]. The results above are in accordance with the results of the cytotoxic effect of pomegranate seed oil on breast cancer cell line MCF-7.
Lansky EP et al., performed an extensive research on pomegranate and its pharmacologic activity. The presence of compounds such as fatty acids including lenolic acid, sterols, steroids, tri-glycerols in pomegranate seed was established. Further, the anticancer, antioxidant and anti-inflammatory properties were proven during this study [4]. According to current study, our results from various tests on two cancer cell lines showed the anticancer properties of pomegranate seed oil.
Limitation
Like any in vitro studies, relating this information to clinical situations must be careful and have to be investigated in the future in different clinical trials.
Conclusion
According to the present study, the presence of anti-cancer compounds in pomegranate seed oil are a reason for preventing and slowing the progression of breast cancer and suggests that pomegranate seed oil can inhibit migration and invasion of breast cancer cell lines and may prevent cells to be metastatic.