The most common form of periodontal disease is gingivitis affecting more that 90% of the world population regardless of age and sex [1]. Gingivitis is a chronic inflammatory disease of gingiva that is characterised by bleeding from gingiva with no attachment or alveolar bone loss [1,2]. Gingivitis if left untreated can progress to periodontitis causing mobility and loss of tooth [3]. Dental plaque is the main aetiological factor responsible for progression of gingivitis to periodontitis [4]. Mechanical plaque control method combined with professional treatment is the most effective and simple method for removal of dental plaque [4]. However, in some patients the lack of compliance causes majority of the patients’to have less than minimum requirements of optimal oral hygiene [5]. To overcome the short comings of mechanical regimen, the utilization of antimicrobial agents have been proven to be useful as an adjunct therapy [3,5].
To the best of our knowledge there is no literature comparing the anti-inflammatory effects of pomegranate gel by assessing the inflammatory cytokine and chemokine levels. Thus, we aimed to evaluate the anti-gingivitis activity clinically and by assessing the inflammatory biomarkers after application of pomegranate extract gel and by comparing it with the commercially available chlorhexidine gluconate gel and combination of chlorhexidine and ornidazole gel as reference drugs.
Materials and Methods
The study was a single-centre, randomized, controlled, parallel group, double-blind clinical trial to test the efficacy of PEG using the two week experimental gingivitis model. The experimental gingivitis model is a gold standard model that has been frequently used as a short term model to evaluate the anti-plaque and anti-gingivitis efficacy of chemotherapeutic agents. The study was conducted in Department of Dentistry, Rajendra Institute of Medical Sciences, Ranchi, India from October 2016 to May 2017. The study protocol was approved by the Institutional Ethics Committee and was registered in Clinical Trial Registry, India (CTRI/2017/08/009559). All the participants were informed about the study and signed an informed consent form in compliance with the study. The study conduct of the principles outlined the Declaration of Helsinki on experimentation involving human subjects were followed.
Sample Size
Sample size was calculated according to a previous report on the deviations seen in CCL28 [15]. On the basis of this data the sample size was calculated to 17 subjects. However, considering the possibility of drop-out among subjects, a total number of 20 patients were selected for each group. The sample size was calculated for an alpha error of 0.05 and power at 90%.
Study Participants and Test Product
Eighty subjects, aged 18-35 years were recruited for this study [Table/Fig-1].
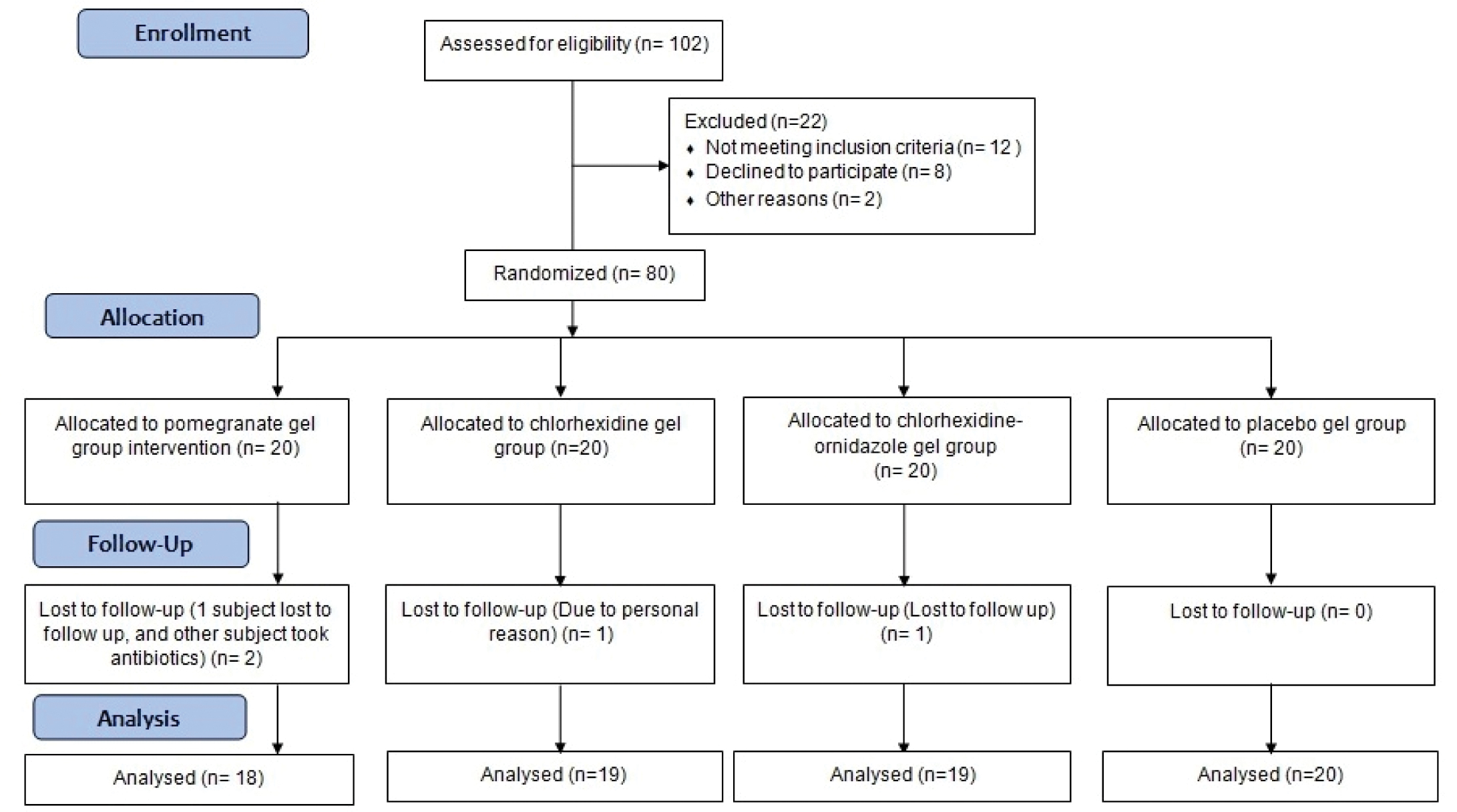
The participants who met the following criteria were included in the study: (i) presence of atleast 20 natural teeth, (ii) exhibit good periodontal health with no teeth having probing depth >3 mm, (iii) a gingival index (GI) [16] and plaque index (PI) ≥1.95 [17], (iv) systemically healthy individual, (v) negative for hypersensitivity or allergy to pomegranate fruit, ornidazole and/or chlorhexidine. Subjects with history of antibiotic and anti-inflammatory usage in the last six months, history of periodontal therapy including oral prophylaxis in the last six months, presence of oral soft tissue pathology, tobacco smokers and/or chewers, pregnant or lactating mothers were excluded from the study. Grossly carious, fully crowned, or restored, orthodontically banded, abutment or third molar teeth were not included in the tooth count.
In this clinical trial two different commercially available chemotherapeutic preparations were tested against pomegranate extract gel:
Group I: 10% Pomegranate extract gel (PEG).
Group II: Gel containing chlorhexidine (CHX) (Hexigel®, ICPA Health Products Ltd, Mumbai, MH, India). Each gram contained Chlorhexidine equivalent to Chlorhexidine Gluconate 1% w/w.
Group III: Gel containing ornidazole and chlorhexidine (CHX-ORD) (Ornigreat gel®, Future Mankind, Mumbai, MH, India). Each gm contained ornidazole (10 mg), and Chlorhexidine Gluconate Solution IP 0.25% w/w.
Group IV: Placebo gel (PG) without any active ingredient.
To ensure proper concealment, all the tubes were similar in size and shape and treatment codes were given to each tube. All the topical gels were packaged in 20 g tube. Except for the principal investigator neither the examiner nor the recorder had access to the subject’s treatment code and patients were also blinded to the treatment product used.
Preparation of Punica Granatum Extract Gel
Fresh pomegranates were obtained from the grocery store and their seeds were separated and ground into fine powder in an electric grinder. A concentrated extract was prepared with powdered material at a ratio of 100 g of powder to 1000 mL of distilled water. To obtain a concentrated extract direct percolation technique was used by filtering the juice in a Buchner funnel through a filter paper. Thereafter, 50 g of carboxymethyl cellulose was added to the 1000 ml of concentrated extract of pomegranate juice and the mixture was boiled until complete dissolution to obtain 10% gel concentration. This preparation was done similar to previous studies [18,19]. Small amounts of menthol and methyl paraben was added as flavouring and preservative agents. The placebo gel had the same composition except for the Punica granatum extract.
Study Design
The clinical trial was divided into three phases; Pre-treatment phase, treatment phase and post-treatment phase.
Pre treatment phase: The study participants were given complete oral prophylaxis to remove supragingival plaque, stain and calculus. Oral hygiene instructions and motivation for maintenance were reinforced by the investigator. A quadrant for the experimentation was randomly selected by lottery method.
Preparation of tooth shield: Upon selection of the experimental quadrant, alginate impression was taken and a cast was prepared by pouring in die stone. A spacer of 0.5 mm was made using a vacuum former. Upon the spacer, an individual tooth shield was made of 2 mm thick thermoplastic mouth guard material using the same vacuum former. The tooth shield was trimmed 2 mm beyond the gingival margin such that the gel would be in contact with the teeth of the experimental quadrant. The tray was also used to prevent brushing of the selected quadrant for induction of experimental gingivitis.
Treatment phase: The participants returned one week after oral prophylaxis. Baseline recording was made for the experimental quadrant and GCF was collected. The participants were continued if they had a GI and PI score of less than 0.5 and absence of BOP. After inclusion the subjects were stratified into four groups, with 20 in each group. The individuals were distributed in a group randomly according to a computer generated code.
An oral hygiene kit was given to each subject containing the tooth shield, 20 g topical gel, commercial dentifrice and a tooth brush. During the two week experimental period, the subjects were instructed to massage the gel into the gingiva uniformly and then fill the tooth shield with the 1 g of gel (2 cm) and seat it over the experimental quadrant for atleast three min. The gel application was done twice daily, once in morning and evening and patients were instructed to refrain from brushing in the experimental quadrant. This was followed by brushing and flossing of the remaining quadrants with the tooth shield placed in the experimental quadrant. The participants were instructed not to consume any food or liquid for at least one hour after gel application. The gel application was unsupervised; therefore each subject was given a diary to record the time and duration of application. Verbal and written instructions were given to each subject to follow at home. They were told to report back in case of any use of antibiotics or anti-inflammatory drugs or occurrence of any side effects, such as pain, itching, ulceration, discomfort, discoloration and taste disturbance.
Post-treatment phase: Following 14 days of gel application, a second recording was made and GCF sample was collected. The subjects received professional scaling and tooth polishing and oral hygiene instruction to re-establish periodontal health. The subjects were followed-up at 30 and 60 days to evaluate the signs of resolution of gingivitis and periodontal health. The clinical recording was done after 60 days. Each subject was given two tubes of 20 g of topical gel. To check for compliance the patients were asked to return the topical gel at the end of 14 days and the remaining amount of gel was measured.
Outcome Measurement
All the recordings were made by a single calibrated periodontist i.e the examiner and recorded by another dentist i.e. the recorder.
The primary outcome assessment of IL-1β, IL-8 and CCL28 levels from GCF was done at baseline and 14 days following treatment for all participants. All the clinical measurements were taken for the experimental quadrant at baseline, 14 days and 60 days. Gingivitis and plaque levels were measured using GI and PI [15, 16]. Measurements were made on six sites per tooth (distobuccal, buccal, mesiobuccal, mesiolingual, lingual, distolingual) on all teeth present. The BOP was recorded as overall percentage of sites with bleeding occurring within 15 seconds of probing. Probing depth (PD) was measured from the free gingival margin to the base of the pocket at the selected sites by using UNC-15 periodontal probe (Hu-Friedy Inc®, Chicago, Illinois, USA). In addition to this examination, the gingiva and the surrounding soft tissue was visually inspected for the presence of any adverse reaction by the same examiner.
Subjective evaluations of the participants were done after 14 days using a questionnaire. A custom made questionnaire was used to evaluate the subjective symptoms after the use of interventional gel. The questionnaire was validated on five patients before the start of the study. The reliability of the questionnaire was estimated using Cronbach’s alpha which yielded a value of 0.853. The subjects were requested to mention yes/no for questions related to pain, burning sensation, pruritis/itchiness, dryness of the mouth, taste disturbance, discolouration of the teeth, and feeling of bitter taste in the mouth. The patients were asked to rate the severity of the side effects as mild, moderate or severe if present.
Examiner Calibration
A single periodontist was trained and calibrated prior to the start of the study. The periodontist examined 10 random subjects initially for GI, PI and PD and then again reassessed after 60 minutes. The examiner’s repeatability of mean index scores was assessed using the intraclass correlation coefficient. The intraclass coefficients for the mean GI, PI and PD were 0.86, 0.91 and 0.89.
Collection of GCF
The GCF collection was done twice (baseline and 14 days) by single periodontist (SN). The baseline collection was done prior to the gel application and second collection was done 14 days after. GCF collection was done from five sites which included mid labial site for central, mesio-buccal site for the premolar, and disto-buccal site for first and second molar. This same site was used for collection each time and the collection was always done prior to clinical assessment. The teeth were isolated with sterile gauze and the areas around the gingival crevice were gently dried with an air syringe. The 1-5 μl calibrated microcapillary pipettes were used for GCF sample collection.
Assessment of IL-1β, IL-8 and CCL28 from GCF
The IL-1β, IL-8 and CCL28 were measured according to manufacturer’s instruction using a commercially available enzyme-linked quantitative sandwich immunosorbent assay (ELISA) kit (Raybio®, Human ELISA kits, Ray Biotech, Inc., USA). Level of cytokine expression was assessed by an ELISA reader at 450 nm. The concentration of the IL-1β, IL-8 and CCL28 were reported as pictogram per millilitre of sample.
Statistical Analysis
The general characteristics of the study population were described using mean±standard deviation (SD). For intragroup comparison paired t-test was used and for intergroup comparison One-way ANOVA followed by post-hoc Tukey’s test was done. The significance level was set at 5%. For data analysis SPSS software (Version 19.0, SPSS Inc., Chicago, IL, USA) was used.
Results
None of the subjects reported any adverse events related to the product use during the course of the study. All the experimental gel had good acceptance except for few subjects using CHX–ORD gel reported with mild bitter and metallic taste of the gel.
Clinical Characteristics of Study Participants [
Table/Fig-2]
Clinical characteristics of study participants. p-value calculated using ANOVA test.
| Parameters | Group I | Group II | Group III | Group IV | p-value |
|---|
| Number of subjects | 18 | 19 | 19 | 20 | 0.18 |
| Age (Mean±SD) | 24.05±4.88 | 22.70±4.73 | 25.35±5.02 | 22.45±4.56 | 0.58 |
| Sex (Male: Female | 11:7 | 10:9 | 8:11 | 9:9 | 0.52 |
| Mean volume of GCF at baseline (μL) | 3.60±0.52 | 3.26±0.76 | 3.48±0.83 | 3.21±0.44 | 0.78 |
| Mean volume of GCF at 14 days (μL) | 3.92±0.93 | 3.96±0.95 | 3.76±0.76 | 3.45±0.42 | 0.23 |
| Total mean gel mass administered (g) | 27.65±4.18 | 27.70±5.18 | 23.70±5.64 | 29.82±5.45 | 0.68 |
Group I- Pomegranate gel, Group II- Chlorhexidine group, Group III- Ornidazole-chlorhexidine, Group IV- Placebo gel. GCF- gingival crevicular fluid, SD- standard deviation.
All the four groups were similar with respect to age and sex. Four participants were excluded from the study; one subject each from group I and III was lost to follow up, one subject from group I needed extraction of third molar and had to take antibiotics and one patient from group II refused to participate due to personal reasons. The amount of gel used by the participants during the course of the study was similar (p=0.68) among the groups. The mean GCF volume collected was non-significant in all the groups at baseline (p=0.78) and 14 days (p=0.23).
Clinical Parameters
[Table/Fig-3] represent the intragroup comparisons of the four groups at different time intervals. In all the groups BOP, PI, GI scores increased from baseline to 14 days (p<0.001) although in group I the increase of BOP and GI scores was less when compared with the other three groups. At day 60 there was a gradual decrease in all the three clinical parameters and no significant difference was observed from baseline to 60 days. The PD remained almost similar at all the time intervals and no significant difference was observed [Table/Fig-3]. The intergroup analysis showed that all the groups were similar at baseline with respect to BOP, PI, GI and PD [Table/Fig-4]. After 14 days significant difference (p<0.001) was noted among the four groups with respect to BOP, PI and GI, except for PD which remained similar in all the groups. When the groups were compared individually for PI, BOP and GI a significant difference was noted at 14 days except for PI scores of PEG group and CHX-ORD groups, which were similar.
Intragroup comparison and mean change from baseline of clinical parameter at different time points for all the groups. Results calculated using paired t-test.
| Parameter | Group | Baseline | 14 days | 60 days | Baseline- 14 days | Baseline- 60 days |
|---|
| Difference | p-value | Difference | p-value |
|---|
| BOP | IIIIIIIV | 0.0±0.00.0±0.00.0±0.00.0±0.0 | 0.08±0.160.61±0.120.33±0.090.82±0.14 | 0.0±0.00.0±0.00.0±0.00.0±0.0 | 0.08±0.160.61±0.120.33±0.090.82±0.14 | 0.01*<0.001**<0.001**<0.001** | 0.0±0.00.0±0.00.0±0.00.0±0.0 | NCNCNCNC |
| PI | IIIIIIIV | 0.14±0.050.19±0.130.18±0.180.13±0.12 | 0.59±0.141.05±0.290.53±0.171.21±0.22 | 0.16±0.230.20±0.170.20±0.130.16±0.09 | 0.45±0.090.86±0.160.35±0.111.08±0.13 | <0.001**<0.001**<0.001**<0.001** | 0.02±0.270.01±0.120.02±0.100.03±0.15 | 0.50 0.700.300.41 |
| GI | IIIIIIIV | 0.41±0.230.31±0.160.29±0.210.21±0.11 | 0.49±0.301.59±0.521.01±0.331.78±0.22 | 0.39±0.340.39±0.180.25±0.240.28±0.14 | 0.45±0.331.28±0.580.72±0.411.57±0.11 | 0.05*<0.001**<0.001**<0.001** | (-)0.05±0.320.08±0.28(-)0.04±0.180.07± 0.16 | 0.500.160.480.25 |
| PD | IIIIIIIV | 2.05±0.482.25±0.392.30±0.572.11±0.20 | 2.18±0.532.29±0.492.32±0.542.15± 0.25 | 2.23±0.522.26±0.312.42±0.542.35±0.20 | 0.14±0.670.05±0.320.02±0.740.04±0.5 | 0.160.270.240.20 | 0.14±0.67(-)0.03± 0.210.10±0.640.20±0.51 | 0.370.280.340.41 |
*statistically significant; ** statistically very significant.
I = pomegranate group; II = chlorhexidine group; III = chlorhexidine-ornidazole group; IV = placebo; GI = Gingival Index; PI = Plaque Index; BOP = Bleeding On Probing; PD = Probing Depth; NC = Not Calculable.
Intergroup comparison of clinical parameter at various follow-up visits from baseline.
| Parameter | Group | Baseline | 14 days | 60 days | Difference |
|---|
| Baseline-14 days | Baseline- 60 days |
|---|
| BOP | I, II, III, IVI vs. III vs. IIIII vs. IIII vs. IVII vs. IVIII vs. IV | 1 | <0.001**<0.001**<0.001**<0.001**<0.001**<0.001**<0.001** | 1 | <0.001**<0.001**<0.001**<0.001**<0.001**<0.001**<0.001** | 1 |
| PI | I, II, III, IVI vs. III vs. IIIII vs. IIII vs. IVII vs. IVIII vs. IV | 0.38 | <0.001**<0.001**0.89<0.001**<0.001**<0.001**<0.001** | 0.58 | <0.001**<0.001**0.96<0.001**<0.001**<0.001**<0.001** | 0.32 |
| GI | I, II, III, IVI vs. III vs. IIIII vs. IIII vs. IVII vs. IVIII vs. IV | 0.09 | <0.001**<0.001**<0.001**<0.001**<0.001**<0.001**<0.001** | 0.44 | <0.001**<0.001**<0.001**<0.001**<0.001**<0.001**<0.001** | 0.36 |
| PD | I, II, IIII vs. III vs. IIIII vs. IIII vs. IVII vs. IVIII vs. IV | 0.53 | 0.71NCNCNCNCNCNC | 0.07 | 0.81NCNCNCNCNCNC | 0.63 |
Only p-values are given here as the individual groups’ MGI, PI, BOP and PD values are given in [Table/Fig-3]. Results are calculated using ANOVA. Pair wise comparison and p-value are done only when comparison by ANOVA is significant.
*statistically significant; ** statistically very significant, NC= Not Calculable as all values were similar. I = pomegrante group; II = chlorhexidine group; III = chlorhexidine-ornidazole group; Group IV = placebo; GI = Gingival Index; PI = Plaque Index; BOP = Bleeding On Probing; PD = Probing Depth.
IL-1β, IL-8 and CCL28 Levels [Table/Fig-5,6]
Among PEG group, the increase in IL-1β and IL-8 from baseline to 14 days was less (p=0.003, 0.002) when compared to CHX (p<0.001, 0.001), CHX-ORD (p<0.001, 0.001), and PG group (p<0.001, 0.001). In PEG group, the CCL28 levels were non-significant when difference was calculated from baseline to 14 days whereas in CHX, CHX-ORD and PEG group there was significant increase (p<0.001, 0.02, <0.001) [Table/Fig-5]. When intergroup comparison was done among the four groups, IL-1β, IL-8 and CCL28 were significant (p <0.001, 0.001, 0.001). When individual group comparison was done a significant difference was observed for the IL-1β and CCL28 and IL-8 levels (p<0.001). The difference was less for PEG group and CHX-ORD for the levels of IL-1β and CCL28 [Table/Fig-6].
Intragroup comparison of cytokines levels from baseline to 14 days.
| Cytokine | Group | Baseline | 14 days | Difference | p-value |
|---|
| IL-1β | IIIIII IV | 21.41±5.2119.93±7.2728.64±7.4029.45±9.28 | 35.28±15.7187.24±15.4248.25±11.24105.54±41.67 | 13.88±8.8767.55±8.8119.63±15.9676.21±38.87 | 0.003*<0.001**<0.001**<0.001** |
| IL-8 | IIIIII IV | 34.23±23.3147.84±45.3731.21±26.4539.54±19.98 | 41.92±17.2198.21±23.7363.59±32.54126.85±22.05 | 7.68±8.2350.37±19.2532.35±12.4787.31±11.36 | 0.002*<0.001**<0.001**<0.001** |
| CCL28 | IIIIII IV | 73.35±9.6575.28±19.2780.58±12.2879.25±11.27 | 80.59±16.94112.68±18.3093.64±25.90139.36±24.10 | 7.12±8.7837.15±12.8213.41±10.6860.13±9.83 | 0.15<0.001**0.02*<0.001** |
p-value calculated using paired t test.
*statistically significant; ** statistically very significant.
I= pomegrante group; II= chlorhexidine group; III= chlorhexidine-ornidazole group; Group IV= placebo.
Intergroup group comparison of cytokine levels from baseline to 29 days. (only p-values) Results for p-value calculated using ANOVA.
| Cytokine | Group | Baseline | 14 days | Difference |
|---|
| IL-1β | I, II, III, IVI vs. III vs. IIIII vs. IIII vs. IVII vs. IVIII vs. IV | 0.86 | <0.001**<0.001**<0.01*<0.001**<0.001**<0.001**<0.001** | <0.001**<0.001**<0.01*<0.001**<0.001**<0.001**<0.001** |
| IL-8 | I, II, III, IVI vs. III vs. IIIII vs. IIII vs. IVII vs. IVIII vs. IV | 0.79 | <0.001**<0.001**<0.001**<0.001**<0.001**<0.001**<0.001** | <0.001**<0.001**<0.001**<0.001**<0.001**<0.001**<0.001** |
| CCL28 | I, II, III, IVI vs. III vs. IIIII vs. IIII vs. IVII vs. IVIII vs. IV | 0.56 | <0.001**<0.001**<0.01*<0.001**<0.001**<0.001**<0.001** | <0.001**<0.001**<0.01*<0.001**<0.001**<0.001**<0.001** |
Only p-values are given here as the individual groups’ cytokine levels are given in [Table/Fig-5].
*statistically significant, ** statistically very significant, I=pomegranate group; II= chlorhexidine group; III= chlorhexidine-ornidazole group; Group IV= placebo.
Discussion
Plaque-induced gingivitis is most common form of periodontal disease causing inflammation of the marginal gingiva. Routine plaque control methods are necessary for maintaining periodontal health and to prevent gingival and periodontal diseases [4] but sometimes these procedures may not be sufficient to maintain gingival health and therefore, adjunctive methods are required [9]. Thus, the use of topical agents can be beneficial as an adjunct therapy [20]. Local application of topical gel has shown greater efficacy than in the form of mouthwash [20,21]. In our study, we selected the two popularly available topical gel, chlorhexidine and ornidazole. Chlorhexidine is the gold standard for subgingival chemical plaque control [22]. Chlorhexidine has been found to be effective against subgingival bacterial flora responsible for the periodontal disease and has no evidence of development of bacterial resistance [21]. It is well retained (substantivity) in the oral cavity and causes no systemic toxicity from local application or ingestion [22]. Ornidazole belongs to the 5-nitro-imidazole group and is similar to metronidazole. Ornidazole has been preferred over metronidazole as the plasma half-life is 1.7 times greater than that of metronidazole when administered locally and requires a low minimum inhibitory concentration to inhibit the growth of periodontal pathogens [23,24]. The side effect of chlorhexidine includes staining of teeth, loss of taste perception and brown discolouration of teeth, while ornidazole can cause bacterial resistance against antibiotics. Due to the drawbacks of the synthetic agents, there has been a shift of focus towards natural and herbal medicaments.
Pomegranate fruit including its peel, seeds, oil, powdered extracts and juice have medicinal property and exhibit no side effects. Pomegranate flavonoids have anti-inflammatory and antibacterial properties and pomegranate polyphenols have antioxidant and antiviral properties [11]. In our study, the PEG gel caused less increase in gingival inflammation and gingival bleeding compared to CHX gel, CHX-ORD gel and PB gel. Somu CA et al., [19] used pomegranate gel as an adjunct to mechanical debridement and similarly observed significant reduction in gingivitis. Similarly Ahuja S et al., [25] and Majumdar M et al., [26] used pomegranate mouthwash for the treatment of gingivitis and observed improvement in BOP and GI. The increase in PI scores was less for PEG gel and CHX-ORD gel. Studies show that rinsing of mouth with pomegranate extract showed capacity to remove dental plaque, increased antioxidant activity, decreased activity of aspartate aminotransferase and proteins [27,28]. The pomegranate mouthwash inhibited major periodontal pathogen, Aggregatibacter actionomycetemcomitans, Porphyromonas gingivalis, and Prevotella intermedia [14,29,30]. Ornidazole gel was equally effective in reducing the PI as it exhibits greater antimicrobial action as it acts against obligate gram negative and gram positive anaerobes [24,31]. Although, in a study by Leishman SJ et al. [32] 10% Punica granatum extract gel was not efficient in preventing plaque formation and gingivitis [32]. The gel was placed into tooth shield in a non-diluted form, and it needed to be solubilised with saliva to exhibit antimicrobial action [29]. In our study topical gels were massaged into the gingiva prior to placement into tooth shield which allows the antiseptic agent to disperse throughout the gingiva and stimulate circulation within the gingival tissues. CHX gel showed improved clinical parameters compared to placebo gel. Several studies have shown the effects of CHX gel in improving BOP, GI, and PI [9,21,22].
To study, the antiinflammatory effects of PEG gel GCF was used for the assessment of the inflammatory cytokines and chemokines. The GCF is an inflammatory exudate produced as a result of inflammation within the gingival tissues and flows continually into the gingival sulcus [32]. Gingivitis is associated with increased GCF levels of inflammatory mediators including IL-1β, IL-8 and CCL28 [9,15,32,33]. These are amongst the most important pro-inflammatory cytokines and chemokine and play a critical role in the destruction of periodontal tissue, alveolar bone and connective tissue [15,32,33]. In our study, we found that there was less increase in IL-1β and IL-8 in participants using PEG gel. The CCL28 level was almost similar to baseline and was not elevated during the experimental gingivitis period. Pomegranate gel was effective in reducing the inflammatory mediators responsible for progression of periodontal disease. Sastravaha G et al., [34] similarly observed decrease in IL-1β and IL-6 after the use of gel with pomegranate extract. Pomegranate also decreased TNF-α and interfered with the activity of elastase, myeloperoxidase and metalloproteinase-3 [14]. The anti-inflammatory mechanism could be explained by the ability of the pomegranate to inhibit NF-κB activity and blocking the cell signalling pathways. Pomegranates seed oil inhibited the key enzymes cyclooxygenase 1, 2 and lipoxygenase as they are responsible for production on various inflammatory mediators [14]. There are no studies that assessed the inflammatory cytokines after the use of ornidazole topical gel, so a direct comparison could not be made. In our previous study we have seen improvements in IL-1β and CCL28 among subjects using metronidazole-chlorhexidine combination gel [9].
In our study we used experimental gingivitis model proposed by Loe H et al., [2] for testing the efficacy of topical gel. The subjects abstained from all mechanical oral hygiene procedures for the two week study period. Similar model was used by Charles CA et al., [35] and Amini P et al., [36]. Better participant adherence and smaller sample size are advantages of short-term experimental gingivitis model [37].
Limitation
All the products were tested in experimental gingivitis model, which may not be similar as naturally occurring gingivitis [38]. A cross over design would have been more suitable to avoid any bias occurring due to variable host response.
Conclusion
Mechanical plaque method is the mainstay of routine hygiene method whereas topical agents only act as an adjunctive aid. Hence, the use of topical anti-gingivitis products would cause reduction in the severity of gingival inflammation rather than a complete prevention or resolution of gingivitis. Punica grantaum gel had showed better anti-inflammatory and anti-gingivitis effects than chlorhexidine and ornidazole gel and was equally effective against dental plaque as ornidazole gel. Pomegranate extract gel could replace synthetic agents as it is safe, non invasive and has no adverse effects.