Prevalence of major congenital anomalies including live births, stillbirths and terminations of pregnancy for foetal anomaly following prenatal diagnosis, is 2-3% [1,2]. Prevention and management of birth defects, particularly in developing and under developed countries has been lacking. A valid concern was raised at the 63rd World Health Assembly that considering the diversity of causes and determinants of congenital disorders, the resources allocated for the same are limited [3].
A congenitally malformed baby results in emotional stress on couples and it is very important to understand the exact aetiology. Epidemiological studies have primarily focused on the role of maternal factors in the causation of birth defects [4]. There is paucity of studies evaluating the contribution of paternal factors. It has been reported that men having sperm with DNA damage have increased incidence of infertility, poor sperm morphology, increased incidence of genetic and epigenetic abnormalities, increased incidence of recurrent spontaneous abortions and malformations in the fetus probably as a result of defective organogenesis [5-7]. Oxidative stress is a major cause of sperm DNA damage and may play havoc with the programmed development of the embryo [8-10]. Studies in assisted reproduction techniques have shown correlation of sperm DNA damage with poor embryo quality [11]. If a probable cause is found for the congenitally malformed foetus, perhaps prevention strategy such as antioxidants can be evolved [12]. In view of this, we designed this pilot study for evaluating the role of seminal oxidative stress and sperm DNA damage in causation of congenital anomalies in the foetus.
Materials and Methods
This prospective study was conducted at the Department of Obstetrics and Gynaecology, in collaboration with the Department of Anatomy and Paediatrics at a tertiary health centre, from September 2012 to May 2013. Ethical clearance was obtained from the Institute Ethical Committee. Informed consent was obtained from all the subjects. It was a pilot study. Convenient sampling was done and an initial sample size of 25 patients were taken.
A total of 25 couples with antenatal ultrasonologically detected foetal congenital anomalies were enrolled, after extensive investigations revealed no maternal or foetal cause of malformations. These investigations included karyotype of partners, thyroid function tests, maternal fasting and post-prandial blood glucose. It was also ensured that there was no history of teratogenic drug intake or radiation exposure in the first trimester. Genetics division was also consulted and an invasive testing for foetal karyotype was performed. For this purpose, amniocentesis was done in 16 cases (64%), followed by cordocentesis in 8 cases (32%), whereas one patient who presented at 33 weeks opted for only post natal evaluation of foetus. History of smoking and alcohol intake were separately recorded for the male partners.
Measurement of Oxidative Stress
Semen analysis was done according to WHO criteria [13]. A 10 μL of 5 mM solution of luminol in DMSO (DimethySulphoxide, Sigma Chemical Co.) was added to 400 uL of liquefied neat semen to measure ROS production. Blank consisted of 10 μL of 5 mM luminol (5-amino-2,3-dihydro- 1,4-phthalazinedione, Sigma Chemical Co., St. Louis, MO, USA), whereas positive control consisted of 25 uL hydrogen peroxide with 10 uL luminol. Single detector luminometer (Sirius, Berthold Detection Systems, USA) was used to assess the levels of ROS by measuring the chemiluminescence. All the samples were measured in duplicate and the average of the readings was taken. Results were expressed in relative light units per second per million spermatozoa.
Sperm Chromatin Structure Assay (SCSA)
1) Preparation of samples: An aliquot of 100 μL of the raw semen was stored at -80°C .SCSA was performed according to the procedure described by Evenson DP et al., [14]. The aliquot from each ejaculate was thawed in a water bath at 37°C for 30 seconds and diluted to a concentration of 2 × 106 sperm/mL in TNE buffer (pH 7.4, working solution, 0.01 M Tris-HCl, 0.15 M NaCl, 1 mM EDTA) to a total of 200 mL in a falcon tube. 0.20 mL aliquots of diluted samples were mixed with 0.40 mL of acid-detergent solution (0.08 M HCl, 0.15 M NaCl, 0.1% Triton X-100, and pH 1.2). After exactly 30 seconds, 1.2 mL of Acridine Orange (AO) staining solution (6 mg AO {chromatographically purified; Polysciences, Inc. USA} per mL citrate buffer {0.037 mol/L citric acid, 0.126 mol/L Na2HPO4, 1.1 mmol/L EDTA disodium, 0.15 mol/L NaCl, pH 6.0}) was added.
2) Flow cytometric measurements [14]: The samples were analysed using a FAC Scan flow cytometer (BD Biosciences), with an air-cooled argon laser operated at 488 nm and a power of 15 mW. The green fluorescence (FL1) was obtained through a 515-545 nm band pass filter, and the red fluorescence (FL3) was obtained through a 650 nm long pass filter. The sheath/ sample was set on ‘‘low,’’ adjusted to a flow rate of 200 events when analysing a sample containing 2 × 106 sperm/mL. Immediately after the addition of the AO staining solution, the sample was placed in the flow cytometer and run through the flow system. All the samples were assessed in duplicate at 2 to 3 week interval and the average was taken.
3) DFI Calculation: At the completion of sample analysis, manual recording of the X-mean (red fluorescence) and Y-mean (green fluorescence) values were done after selecting gate for sperm cells using Flow Jo software (Oregon, USA). DNA Fragmentation Index (DFI) was used to express the extent of DNA denaturation using formula;
DFI = Mean Red Fluorescence/ (Mean Red Fluorescence + Mean Green Fluorescence) [14].
Statistical Analysis
Statistical analysis was done using Statistical Package for Social Sciences (SPSS) IBM Version 19.0. Descriptive statistics such as mean, median, standard deviation and range were calculated for semen ROS and sperm DFI. Normality test was done using Shapiro- Wilk test. Non-parametric test (Mann-Whitney test) was used for comparing median ROS and DFI. Bivariate correlation analysis was carried out to find if there is significant correlation between Semen ROS and Sperm DFI. For statistical significance probability of p <0.05 was considered.
Results
The most common foetal malformation was neural tube defect, seen in 6 (24%) out of 25, closely followed by bladder outlet obstruction and cystic adenomatoid malformation with 5(20%) and 4 (16%) cases respectively. Other less common malformations with two cases each were mesenteric cyst, ventricular septal defect and multiple anomalies whereas one case each of omphalocele, congenital diaphragmatic hernia, foetal neck mass and skeletal dysplasia was also seen [Table/Fig-1].
Spectrum of foetal malformations in cases.
| Foetal congenital malformation | Number (n=25) | Percentage (%) |
|---|
| Neural tube defects | 6 | 24 |
| Bladder outlet obstruction ± Hydronephrosis | 5 | 20 |
| Cystic Adenomatoid Malformation | 4 | 16 |
| Mesenteric cyst | 2 | 8 |
| Ventricular Septal Defect | 2 | 8 |
| Omphalocele | 1 | 4 |
| Congenital diaphragmatic hernia | 1 | 4 |
| Foetal neck mass | 1 | 4 |
| Skeletal dysplasia | 1 | 4 |
| Multiple congenital anomalies | 2 | 8 |
Among the 25 couples enrolled, the mean age ± SD of the male partners was 30.16±4.03 years. Two out of 25 were smokers and one person had the habit of alcohol consumption. Distribution of semen ROS and sperm DFI was not found to be normal. Therefore, median and IQR was computed.
The median (IQR) semen ROS levels in male partners of the cases was 10.70 (86) RLU/sec/million sperm. At our laboratory, the normal reference value of semen ROS levels has been computed to be < 22.0 RLU/sec/million sperm [15]. Accordingly, 10 (40%) subjects had higher values than the reference value. Median (IQR) values of semen ROS by time since organogenesis is presented in [Table/Fig-2].
Variation of median ROS levels with time since organogenesis (weeks).
| Time since organogenesis(weeks) | Cases (n=25) | Semen ROS levels (RLU/sec/million sperm)Median (IQR)* |
|---|
| 1-10 weeks | 9 | 27.65 (9.2) |
| >10 weeks | 16 | 40.8(14.0) |
*p=0.276 by Mann-Whitney U test.
Semen ROS levels showed weak positive although no significant correlation with age of the male subject amongst the cases (r= 0.204, p=0.327).
The median (IQR) value of DFI (expressed as percentage) was 31.84(13).
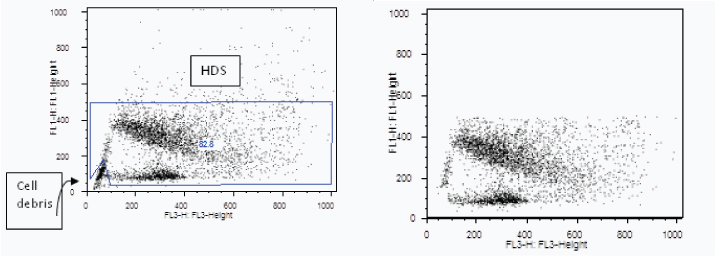
Accordingly, 17(68%) subjects had values more than the reference level. Representative cytograms of high sperm DNA damage are shown in [Table/Fig-3].
(a) Representative cytogram of semen sample of a case showing high DNA fragmentation by SCSA, as the dot plot is widespread towards X-axis. X –axis represents fragmented DNA and Y -axis represents native DNA; (b) Dot plot cytogram of same case semen sample after gating i.e. excluding high DNA stainability cells (marked as HDS on the first plot) and cellular debris
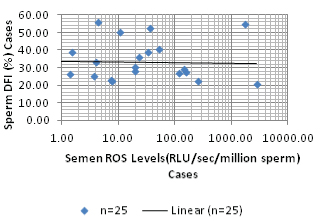
The sperm DFI showed weak positive correlation with age of the subject. (r=0.01, p=0.962). Sperm DFI showed a negative correlation with semen ROS levels (r=-0.033, p=0.88) as shown in [Table/Fig-4].
Correlation between Sperm DFI and Semen ROS.
Discussion
Congenital malformations are a major source of anxiety for the parents as well as a burden on the national resources. Therefore, it is important to understand the aetiology of such malformations, so as to be able to make efforts for their prevention. It has long been understood that DNA damage in the male germ line and free radicals in semen adversely affect fertility potential of couples and affects Assisted Reproductive Technique (ART) outcome [5,6]. The detrimental role of semen oxidative stress in causing sperm DNA damage has also been reported to impair embryo development and increase rates of pregnancy loss [16-18]. Previous study had shown that semen ROS levels are elevated in recurrent abortion cases [19]. Few studies have reported that DFI threshold in infertile cases is approximately 30% whereas a threshold value of 23.54 was obtained to discriminate recurrent pregnancy loss cases from normal controls [9,20,21]. Thus, it may be understood that cases with more than 30% DFI are infertile whereas, cases with lower DFI (approximately 24%) are able to initiate pregnancy, but such cases i.e., DFI between 24 and 30 incur DNA damage enough to adversely alter embryogenesis leading to either pregnancy losses or foetal malformations, genetic and epigenetic abnormalities and increased risk of childhood cancers [7]. However, there are no published studies till date, implicating these sperm factors in causation of unexplained foetal malformations. Therefore, this pilot study was undertaken to evaluate the semen oxidative stress and quality of sperm chromatin structure in men with unexplained foetal anomalies.
In a study by Desai N et al., the best cut-off value of semen ROS to distinguish healthy fertile donors from infertile men was 1.85 × 104 counted photons per minute/20 million sperm [22]. At this threshold, the specificity was 82% and the sensitivity was 78%. This value can be defined as basal reactive oxygen species level in infertile men. At our institute, the reactive oxidative species are reported as Relative Light Units (RLU)/ sec/million sperm, as already mentioned earlier. The conversion factor of cpm/20 million sperm to RLU/sec/million sperm is given by the following:
{1 cpm / 20 million sperm/mL = 8.3 × 10-3 RLU /sec/million sperm}
The normal reference value for ROS is < 22 RLU/sec/million sperm and has already been determined and validated in earlier studies at our institute [15]. In the current study, the median ROS value for our subjects was 10.70 RLU/sec/million sperm. The median values were taken for comparison as the data was highly skewed and sample size was small. Amongst the subjects, only 10 (40%) subjects had higher values than the reference value of <22 RLU/sec/million sperm.
Semen ROS levels showed an trend towards increase with increasing age of the male partner (r=0.204, p=0.327). This result was in concordance with Coccuza M et al., who prospectively evaluated 98 fertile men who were candidates for vasectomy and also observed a positive correlation between seminal ROS levels and age (r=0.20; p=0.040) [23].
Evidence has been accumulating to suggest that sperm nuclear DNA integrity should also be routinely examined in cases of adverse pregnancy outcomes. Several techniques exist to detect sperm DNA fragmentation and extensive basic and clinical research shows SCSA is perhaps, the gold standard. DNA fragmentation index determined by SCSA was used for quantifying the sperm DNA damage in our study. The median (IQR) value of sperm DFI was 31.84 (13)%. Various studies have considered different threshold values for sperm DFI, in causing infertility, recurrent pregnancy losses or adverse outcomes in ART. As discussed, DFI >30% has been associated with infertility, whereas >24% allowed conception, but increased the chance of abortion [9,20,21]. Eight cases (32%) had sperm DFI more than 27% and sperm DFI was seen to increase with the age of the male partner, though statistically not significant (r=0.01, p=0.962). Fang L et al., randomly selected one hundred and eleven infertile couples planned for IVF and also found significantly positive correlation, between sperm DFI and the age of male (r=0.624, p<0.05) [24]. Oxidative stress results in generation of 8-hydroxy-2-deoxyguanisine, a highly mutagenic molecule, which causes single and double strand breaks in sperm DNA especially in sperm telomeres. Telomeres are guardians of our genome and regulate replicative capacity of cells and maintain genome integrity and chromosome stability. Thus, accumulation of breaks in sperm telomeres causes their accelerated shortening and may result in aneuploidies and rearrangements which may lead to congenital malformations [25]. In another study from our laboratory, we have shown a linear positive correlation between seminal ROS and 8-hydroxy-2- deoxyguanosine levels [26].
In a recently published review of 28 studies (8 IVF, 12 ICSI and 8 mixed IVF-ICSI studies) by Zini A et al., evaluated the relationship between sperm DNA damage and embryo quality in artificial reproductive techniques [11]. In 11 of the 28 studies (1/ 8 IVF, 5/12 ICSI and 5/8 mixed IVF-ICSI studies), sperm DNA damage was associated with poor embryo quality and/or development, whereas the remaining 17 studies showed no relationship between sperm DNA damage and embryo quality and/or development. They also stated that there is no consistent relationship between sperm DNA damage and embryo quality and/or development. Our study also reiterates similar conclusion.
Shamsi MB et al., evaluated male partners of 25 cases of recurrent spontaneous abortions and 20 controls for semen oxidative stress and sperm DNA damage [19]. ROS in neat semen was elevated in 62.5% cases, with the mean value of 55,096.4 RLU/min/20 million sperms (~45.91 RLU/sec/million sperm). But in our study, ROS were elevated in only 40% cases with a median of 10.7 RLU/sec/million sperm.
Sperm genome is organised into two fractions: a central protamine bound fraction and the peripheral histone bound fraction. Unfortunately, this peripheral loosely bound chromatin which harbors crucial genomic information is highly susceptible to damage by adverse environment especially oxidative stress. But oxidative stress is only one of the mechanisms of DNA damage. Probably there is a need to analyse other factors like level of sperm transcripts and other regulatory RNA, which might explain why a consistent relation between oxidative stress induced DNA damage and foetal malformation has not been found in this study. These long, non-coding RNA are found mainly around development genes and thus regulate the expression of these genes. As the peripheral portion of sperm genome has genes which remain transcriptionally active these may be most vulnerable to oxidative damage and thus the role of sperm factors especially oxidative stress and DNA damage needs to be further validated [27].
Limitation
Firstly, the sample size was small. Secondly, there is individual variation in the semen oxidative levels with time. The semen oxidative stress and DNA damage was measured seperately from the actual time of organogenesis. Spermatogenic cycle is about 72 days and we do not know the age of the sperm that resulted in successful fertilization. Therefore, these values may not be reflective of the oxidative stress and consequent sperm DNA damage experienced at the time of fertilization and embryo development.
Conclusion
A trend towards higher levels of semen oxidative stress and sperm DNA damage in such cases was found. Further investigation with measurements closer to period of fertilization and adequate sample size is warranted, to gather more evidence. Importantly, if a causal relationship is proved between semen oxidative stress, DNA damage and unexplained foetal malformation, simple preventive strategies like lifestyle modification and antioxidant supplementation can help a lot of distressed couples.
*p=0.276 by Mann-Whitney U test.