The nasal cavity and the paranasal sinuses-including the maxillary, ethmoid, sphenoid and frontal sinuses are collectively termed as the “sinonasal tract” which is anatomically and embryologically distinct from nasopharynx. Neoplasms arising from these structures show similar histological variants and differ only with regards to location and clinical symptomatology [1]. They are one of the most common pathologies of the head and neck. Most of the sinonasal lesions are inflammatory with neoplasms comprising of 3% of all head and neck tumours and <1% (0.2-0.8%) of all malignant tumours [2]. But higher rates are recorded in Japan and certain parts of China and India [3].
Identification of risk factors for sinonasal neoplasm and elucidating the changes in epithelium that precede the development of malignancy is the matter of interest for the past few decades. Risk factors like wood dust and other occupational exposure have been associated with the development of certain sinonasal cancers, like a rare subtype of intestinal type adenocarcinomas. Identification of human papilloma virus has been associated with both neoplastic and non-neoplastic tumours of sinonasal tract particularly in Schneiderian papilloma. However, detection rate ranges from 0-100% [4].
A number of potentially useful biomarkers are being investigated for this purpose, and these include p16 INK4a, HPV E6/E7 mRNA and novel methylation assay. p16 is a CDK-inhibitor tumour suppressor protein known to be overexpressed in HPV-positive head and neck squamous cell carcinomas. The Rb pathway deregulation is brought by the overexpression of E7, which in turn activates the negative feedback loop and leads to cellular accumulation of p16. Thus, it is used as a surrogate marker of HPV [5].
Ki-67 is a cell-cycle associated human nuclear protein which is expressed with cell proliferation and thus widely used in pathology as a proliferation marker to measure the growth fraction of cells in human tumours [6].
Thus, the purpose of this study was to detect the expression of cell cycle protein p16 and cell nuclear proliferating marker Ki-67 in squamous cell carcinoma and its precursor sinonasal lesions using immunohistochemistry.
Materials and Methods
An observational and cross-sectional study was carried out after approval by the Ethics committee in a tertiary care hospital, in the Department of Pathology. This study included 87 patients attending the Department of Otorhinolaryngology with complaints suggestive of masses arising from the sinonasal tract.
Biopsies were taken from all these and the specimens were fixed with 10% neutral buffered formalin, and sections were processed by conventional methods. Original diagnoses were obtained on the initial H&E slides. Special stain Periodic Acid-Schiff (PAS) was done to demonstrate fungal organisms in sections of non-neoplastic masses.
Sinonasal squamous lesions were categorized as inverted papilloma, exophytic papilloma, squamous cell carcinoma arising in pre-existing inverted papilloma, squamous cell carcinoma and sinonasal undifferentiated carcinoma.
Immunohistochemistry
Suitable blocks of neoplastic masses were selected and 4μm sections were cut for further immunohistochemical staining using Ki-67 (Novocastra ready-to-use monoclonal mouse anti Ki-67 antibody-RTU-Ki67-MM1) and p16 (Biogenex monoclonal mouse anti-p16 antibody -G175-405) antibody. Positive and negative controls were stained simultaneously.
Evaluation of Ki-67 staining: Only nuclear staining of epithelial cell was considered.
Intensity of immunohistochemical staining- It was assessed subjectively based on brown colour exhibited by antigen, antibody and chromogen complex. It was graded as follows: Negative (no colour), Mild (light brown colour), Moderate (dark brown colour), Intense (very dark brown colour) [Table/Fig-1].
Figure showing different intensity and distribution of Ki-67: a) moderate intensity (10X); b,c) intense staining (10X); d. mild intensity (40X).
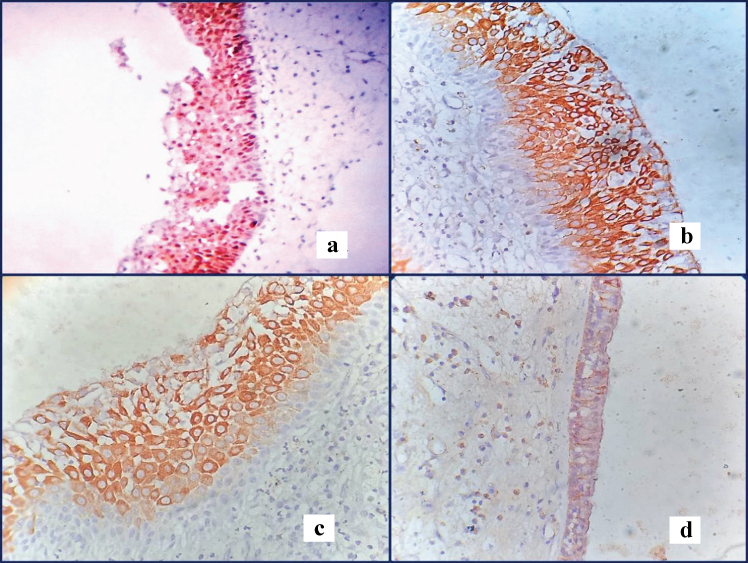
Distribution of staining: It was graded as: confined to only basal layer, both basal and suprabasal layer or all layers of epithelium.
Expression pattern of staining- It was analysed semi-quantitatively as number of positive cells per 100 basal or para basal cells and was finally recorded as percentage: 0-5%=0, 6-25%=2, 26-60%=4, 61-99%=6 [6].
Ki-67 labelling index (Ki-67 LI) measures accurately ki-67 positive tumour cells.
Scoring of p16 expression: Positive when strong and diffuse nuclear and cytoplasmic staining was seen in 70% tumour cells [4].
Statistical Analysis
Chi-square test and One-way ANOVA were used as statistical test to establish difference in mean between different groups and more than two groups respectively. The difference in values was considered significant when p<0.05. Degree of agreement between p16 and Ki-67 was assessed by kappa statistics.
Results
A total of 87 cases of sinonasal masses were received over a period of 18 months. Among these, 39(44.83%) non-neoplastic lesions, 21(24.14%) benign neoplastic lesions and 27(31.03%) malignant neoplastic lesions were found [Table/Fig-2]. Among 39 non-neoplastic cases, a majority of cases (33) were inflammatory polyps. Among 21 benign neoplastic cases, 7 cases were nasal papillomas. Out of total 27 malignant neoplastic cases, majority of cases (8) were squamous cell carcinoma.
Table showing distribution of all sinonasal lesions.
| Broad Category | Histological Diagnosis | Number of Cases (N=87) | Percentage (N=%) |
|---|
| Non-Neoplastic Lesions | Inflammatory polyp | 33 | 37.93 |
| Rhinosporidosis | 4 | 4.60 |
| Actinomycosis | 1 | 1.15 |
| Rhinoscleroma | 1 | 1.15 |
| Benign Neoplastic Lesions | Angiomatous polyp | 6 | 6.90 |
| Angiofibroma | 3 | 3.45 |
| Capillary haemangioma | 1 | 1.15 |
| Glomangiopericytoma | 1 | 1.15 |
| Sinonasal papilloma | 7 | 8.05 |
| Nasal glioma | 1 | 1.15 |
| Pleomorphic adenoma | 1 | 1.15 |
| Fibrous dysplasia | 1 | 1.15 |
| Malignant Neoplastic Lesions | Squamous cell carcinoma | 8 | 9.20 |
| Low-grade transitional carcinoma | 1 | 1.15 |
| Sinonasal undifferentiated carcinoma | 2 | 2.30 |
| Undifferentiated nasopharyngeal carcinoma | 3 | 3.45 |
| Intestinal-type adenocarcinomas | 1 | 1.15 |
| Non Hodgkin lymphoma | 3 | 3.45 |
| Myeloid sarcoma | 1 | 1.15 |
| Primitive neuroectodermal tumour | 3 | 3.45 |
| Olfactory Neuroblastoma | 2 | 2.30 |
| Mucosal malignant melanoma | 1 | 1.15 |
| Adenoid cystic carcinoma | 1 | 1.15 |
| Rhabdomyosarcoma | 1 | 1.15 |
A male predominance (69%) was seen both in the non-neoplastic and neoplastic categories. Both non-neoplastic and neoplastic sinonasal lesions occurred in the age group of 41-50 years. Most of the patients presented with nasal obstruction (89.7%) followed by epistaxis (64.4%) and rhinorrhea (35.6%). Most of the sinonasal lesions were unilateral (73%).
Majority of the sinonasal lesions showed both nasal cavity and paranasal sinus involvement (47%) followed by only nasal cavity involvement (40%). Among the paranasal sinuses, maxillary sinus was most commonly involved.
Ki-67 expression [Table/Fig-3,4, and 5]
Table showing distribution of intensity of staining of Ki-67 in squamous sinonasal lesions.
| INTENSITY | IfP | IvP | EP | IvP with SCC | SCC | SNUC |
|---|
| Negative | 2 | 0 | 0 | 0 | 0 | 0 |
| Mild | 18 | 3 | 0 | 0 | 0 | 0 |
| Moderate | 5 | 2 | 1 | 2 | 0 | 0 |
| Intense | 8 | 1 | 0 | 0 | 6 | 2 |
| Total | 33 | 6 | 1 | 2 | 6 | 2 |
| ς2=31.823, p=0.0068* | | | | | | |
Table showing distribution of pattern of staining for Ki-67 antibody in squamous sinonasal lesions.
| STAINING | IfP | IvP | EP | IvP with SCC | SCC | SNUC |
|---|
| Negative | 2 | 0 | 0 | 0 | 0 | 0 |
| Basal | 31 | 4 | 0 | 0 | 0 | 0 |
| Basal +Suprabasal | 0 | 2 | 1 | 2 | 0 | 0 |
| All layers | 0 | 0 | 0 | 0 | 6 | 2 |
| Total | 33 | 6 | 1 | 2 | 6 | 2 |
| ς2=85.108, p=<0.0001* | | | | | | |
Table showing distribution of labelling index of Ki-67 in squamous sinonasal lesions.
| STAINING | IfP | IvP | EP | IvP with SCC | SCC | SNUC |
|---|
| 0-5% | 23 | 0 | 0 | 0 | 0 | 0 |
| 6-25% | 10 | 4 | 0 | 0 | 0 | 0 |
| 26-60% | 0 | 2 | 1 | 1 | 0 | 0 |
| 61-99% | 0 | 0 | 0 | 1 | 6 | 2 |
| Total | 33 | 6 | 1 | 2 | 6 | 2 |
| ς2=79.500, p=<0.0001* | | | | | | |
a) Inflammatory Polyp (IfP): Staining was mostly mild (18 cases), eight cases showed intense staining and it was limited only in the basal layer with a mean Labelling Index (LI) of 3.82 and Standard Deviation (SD) 4.97. Two cases were negative.
b) Inverted Papilloma (IvP): Four cases showed only basal staining and two cases showed both basal and suprabasal staining. Staining was mostly mild (three cases) and LI ranged from 6-60%. Mean LI was 26 with SD of 10.49.
c) Exophytic Papilloma (EP): Only one case was found that showed moderate basal and supra basal staining with LI of 40.
d) Inverted Papilloma with Squamous cell carcinoma (IvP with SCC): Two cases showed moderate basal and supra basal staining with mean LI of 60 and SD 2.82/
e) Squamous Cell Carcinoma (SCC): All the six cases showed intense staining of all the layers with LI ranging from 65-85%. Mean LI was 75 and SD was 7.07.
f) Sinonasal Undifferentiated Carcinoma (SNUC): All two cases showed intense full thickness staining with mean LI of 85 and SD 4.24.
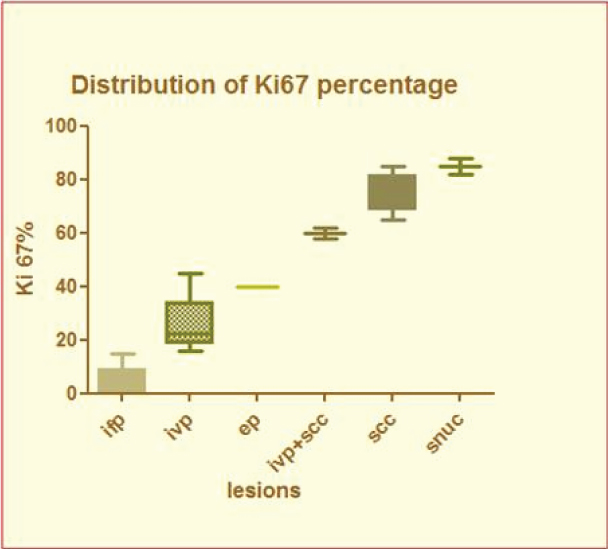
Ki-67 labelling Index was gradually increasing from IfP (3.82%) towards SNUC (85%). Box-and-whisker plot showing Ki-67% is presented as [Table/Fig-6].
Box-and-whisker plot showing distribution of Ki-67 percentage.
p16 expression [Table/Fig-7,8]
Distribution of p16 expression in squamous sinonasal lesions.
| p16 | IfP | IvP | EP | IvP+SCC | SCC | SNUC |
|---|
| Positive | 2 | 2 | 1 | 1 | 2 | 1 |
| Negative | 31 | 4 | 0 | 1 | 4 | 1 |
a) negative p16 expression (40X); b) positive p16 expression (40X).
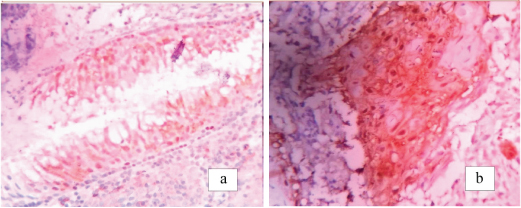
Expression of p16 was highest in both IvP with SCC group and SNUC group compared to 33.3% in IvP group and SCC group.
Correlation between p16 and Ki-67 showed moderate agreement (composite K=0.53) calculated according to Wilson-efficient score method.
Discussion
Sinonasal neoplasms are rare tumours overall. Very few studies have dealt with the correlation of its biological behaviour and histological features. Most of the squamous lesions follow a benign course for a long duration before malignant transformation. Till date, only few specific biomarkers like p53, Rb and Ki-67 that can predict the malignant transformation of these squamous lesions [7]. Various studies reveal that HPV infection is one of the important factors in the aetiopathogenesis of inverted papilloma. The progression of productive high risk HPV infection to the neoplastic process involves an array of changes in the viral gene expression. A number of potentially useful biomarkers are being investigated for this purpose and they include p16 INK4a, HPV E6/E7 mRNA and noble methylation assay [8]. p16 and Ki-67 were used in our study to recognize the histologically significant lesions and role of HPV in its aetiology.
Out of 87 cases of sinonasal masses, 39(44.83%) non-neoplastic lesions, 21(24.14%) benign neoplastic lesions and 27(31.03%) malignant neoplastic lesions were found. Among all lesions, inflammatory polyp (37.93% of total 87 cases) was the commonest. which corroborated with the study done by Zafar U et al., [9]. Most of the lesions have male predominance as supported by Bhattacharya J et al., [10]. The mean age of presentation in our study was 38.2 years which is consistent with 39.4 years observed in other study [11]. Most of the patients presented nasal obstruction (85.06%) followed by epistaxis (60.92%) and rhinorrhea (34.48%). Similar result was documented in other study [11].
Though sinonasal epithelium is an uncommon site for neoplastic processes, still wide range of both epithelial and non-epithelial tumours occur here. Epithelial tumours are three times more common than non-epithelial tumours [12]. But in our study out of 48 neoplastic cases we found 24 epithelial cases (50%) and 24 non-epithelial cases.
Expression of p16 tumour suppressor protein has been proposed to be a surrogate marker for HPV infection [13]. Thus, p16 over expression is thought to mark the presence of biologically active HPV infection [8]. However Smith EM et al., found no concordance between p16 expression and presence of HPV infection in 20% head & neck cancers [14]. In our study, positive p16 expression was observed in 50% cases of SCC arising in pre-existing IvP. But individually IvP and SCC revealed positive p16 expession in 33.3% cases only. Yamashita et al., showed presence of Human papilloma virus infection in the sinonasal region in approximately 37.8% of IvPs and 27% of SCCs. This can conclude that HPV infection can induce malignant transformation of IvP by alternating the expression of cell cycle proteins. Only 1 case of EP showed positive p16 expression. Positive p16 expression was seen in 2 out of 33 cases of IfP as documented in previous study [8].
Ki-67 is a nuclear proliferative marker and labeling index of Ki-67 was highest in SNUC group followed by SCC and IvP with SCC group. A gradual increase in LI of Ki-67 was noted from non-neoplastic IfP group to benign neoplastic IvP group to malignant neoplastic SCC group. A total of 31 out of 33 cases of IfP showed positive basal epithelial layer staining due to physiologically active proliferation of basal layer, but the rest of neoplastic lesions show basal, suprabasal or all layer Ki-67 expression. This concluded that epithelial proliferation increases as the lesion becomes more malignant. Study by Mumbuc et al., showed that both epithelial cell proliferation and Ki-67 LI was higher in IvP group than IfP group [15].
As Ki-67 is expressed in all proliferating cells, it is not a specific marker for neoplastic transformation. On the other hand, p16 is expressed specifically in the epithelium that underwent malignant transformation due to HPV infection. We found moderate agreement (composite K=0.53) between these two biomarkers.
Limitation
1. As squamous sinonasal lesions are rare, our study included only 17 such cases.
2. HPV DNA study could not be done, in positive p16 expression cases, due to lack of resources.
Conclusion
Simultaneous evaluation of p16 expression and cell cycle proliferation marker (Ki-67) revealed significant difference in squamous sinonasal lesions and its precursor lesions. Thus, HPV plays a pivotal role in oncogenesis and malignant transformation in squamous sinonasal lesions.