Incidence and associated mortality due to candidemia is influenced by many factors such as population at risk, standard of the health care facilities available distribution of Candida species and prevalence of antifungal resistance [1,2]. Drugs such as Fluconazole or voriconazole are commonly used since amphotericin B may be quite toxic [2]. According to various recent studies the spectrum of candidemia has changed with the emergence of non-albicans Candida species, the incidence of which in neonates is not well known in our settings [1]. Although Candidaalbicans remains the most common fungal agent from neonatal candidemia, many studies have detected a shift toward non-albicansCandida (NAC) species. Neonatal Candida septicaemia and NAC have been diagnosed with increasing frequency in recent years [1-6].
By our current study useful information for our remote area could be arrived at by comparing the incidence of candidemia due to different species and knowing their susceptibility pattern, since local epidemiological data is crucial in the management of such invasive infections [5,6]; this would not only improve the outcome by guiding to choose appropriate antifungal therapy in neonates and low birth weight babies predisposed to fungal infections but could also to reduce their occurrence.
Materials and Methods
Our study was a prospective cross-sectional time based study conducted on 109 Candida bloodstream isolates (from 109 cases) obtained from all 689 neonates admitted to NICU with suspected bloodstream infections (septicemia) to NICU of New Duffrin Hospital and Shri Chaitanya Hospital Sagar MP, India for a 3 year period from January 2013 to December 2015. All clinically suspected cases of neonatal septicaemia admitted to the NICU were included in the study; neonates admitted for other reasons were not included in the study. The presentation on admission was mainly raised temperature/hypothermia, lethargy, respiratory distress, bradycardia, vomiting, refusal to feed, abdominal distention, jaundice, and referred patients for raised C reactive protein. Consent was obtained from the parent/ guardian regarding use of data in the study and permission taken from institutional ethics committee. As per previously defined criteria they were noted for birth weight, age at admission, administration of broad spectrum antibiotics and prematurity [1,2]. Blood cultures were collected using full aseptic precautions and 2-2.5 ml blood obtained from peripheral veins and culture performed by using aerobic bottles of BACT/ALERT 3D 60 (Biomerieux) automated system. When growth was detected by the instrument subculture was performed on Blood agar, MacConkey’s agar and Brain heart infusion agar. All the Candida bloodstream isolates were identified to species level by using the VITEK2 (Biomerieux) yeast identification system which is a fully automated instrument for identification of microorganisms [8] and were reconfirmed by standard mycology techniques namely- germ tube test, Candida Hichrom (Hi-Media Laboratories, Mumbai, India) agar [9] and morphology of growth obtained on corn meal agar. Susceptibilities were determined by VITEK2 YST card [8] of the VITEK2 (Biomerieux) fully automated instrument which tests minimum inhibitory concentrations (MICS) for Fluconazole, 5 Flucytocine, Amphotericin B, and Voriconazole. The interpretation guidelines used by the instrument software was updated CLSI 2012 guidelines [10]. Statistical analysis was performed by applying chi-square test for incidence of Candidemia among normal birth weight LBW and VLBW neonates, p-value of less than 0.05 was considered significant.
Results
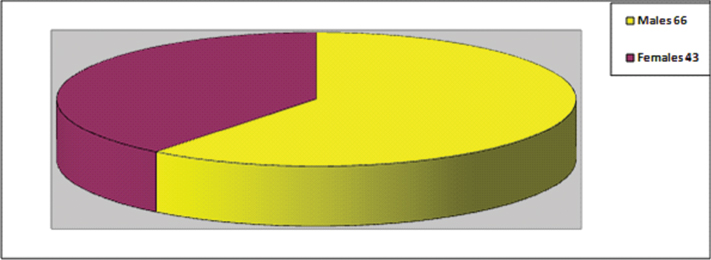
In all, 109 neonatal bloodstream yeast isolates (15.8%) were obtained from of 689 suspected bloodstream infection (septicaemia) cases; total 413 (59.9%) were culture positive, various bacteria were isolated from 304 (44.1%) such cases, no cases of co infection with bacteria and yeast were seen. Yeast isolates showed higher incidence in male neonates as compared to females; Male neonates were 66 (60.5%) in number as compared to 43 (39.5%) females, the sex distribution is shown in [Table/Fig-1].
Sex distribution of Neonates with Candida septicaemia.
A total 45 of these neonates were premature. When birth weight was analysed 34 were low birth weight (LBW), and among these total 29 were both premature and low birth weight. Twenty-two neonates were Very Low Birth Weight (VLBW) with 19 premature and 7 were extremely low birth weight as shown in [Table/Fig-2]. Of 109 neonates having Candida septicaemia all of them were receiving broad spectrum antibiotics, 78(71.5%) were normal deliveries and 31(28.5%) were delivered by cesarean section. The mean time for presentation of Candida septicaemia was age of 9.6 days.
Birth weight distribution of blood culture positive neonates with Bacterial and Candida sepsis.
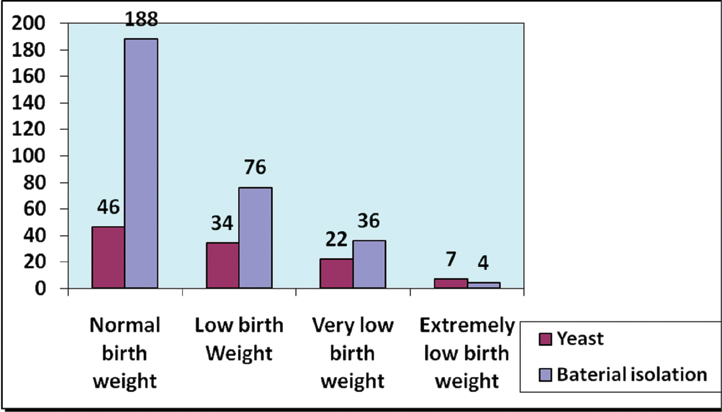
On statistical analysis as shown in [Table/Fig-3],
Statistical analysis for correlation of birth weight with Candida bloodstream infection.
| Normal Wt | LBW | VLBW | Extremely LBW |
|---|
| Yeast | 46 | 34 | 22 | 7 |
| Bacterial | 188 | 76 | 36 | 4 |
| Chi= 18.44, p= 0.0004, Significant |
It was found by application of chi square test to all the culture positive neonates that association of LBW, VLBW or extremely low birth weight association with Candida sepsis was statistically highly significant with P-value of 0.0004. The isolation rates of various Candida species is shown in [Table/Fig-4].
Species distribution of Candida isolates.
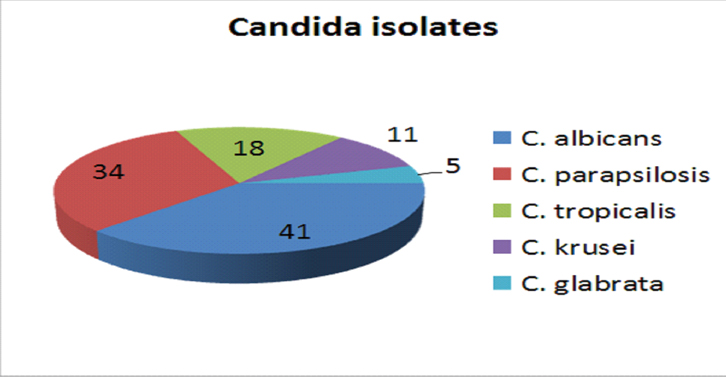
Candidaalbicans was most predominant species with 41 (37.6%) isolates followed by Candidaparapsilosis 34 (31.1%), Candidatropicalis 18 (16.5%), Candidakrusei 11 (10.1%) and Candidaglabrata 5 (4.5%). Overall NAC accounted for 68 (62.4%) of all bloodstream Candida infections. The species identification performed by VITEK2 YST card by the instrument was mostly in agreement when reconfirmed with conventional methods. Only 1 isolate of C. albicans was misidentified (wrongly as C. tropicalis) and 2 other isolates of C. albicans were identified with low discrimination. Identification and susceptibility were completed in much lesser time by VITEK2 in around 24-36 hours, while reconfirmation with standard mycology techniques required 48-72 hours.
The antifungal resistance pattern of various species of Candida isolates obtained by the automated instrument has been shown in [Table/Fig-5].
Anti Fungal Susceptibility of Candida Isolates.
| Antifungal agent | C. albicans n = 41 (resistant%) | C. parasilosis n = 34 (resistant%) | C. tropicalis n = 18 (resistant%) | C. Krusei n = 11 (resistant%) | C. glabrata n = 5 (resistant%) |
|---|
| Fluconazole | 28 (68.3%) | 8 (23.5%) | 9 (50%) | 11 (100%) | 2(40%) |
| 5Flucytocine | 6 (14.6%) | 5 (14.7%) | 6 (33.3%) | 11 (100%) | 1(20%) |
| Amphotericin B | 0 | 3 (8.8%) | 0 | 2 (18.1%) | 0 |
| Voriconazole | 0 | 0 | 0 | 1 (9.1%) | 0 |
All C. albicans, C. tropicalis and C. glabrata isolates (100%) were susceptible to amphotericin B; in these species no resistance (0%) was seen. Out of total 34 isolates of C. parapsilosis tested, only 3 (8.8%) and total 11 C. krusei isolates tested only 2 (18.1%) isolates exhibited an MIC of >1 μg/ ml for amphotericin B. Resistance to fluconazole was observed in 28 (68.3%) of C. albicans, all 11 (100%) C. krusei isolates, 9 (50%) of C. tropicalis, 2 (40%) C. glabrata isolates and 8 (23.5%) of C. parapsilosis, all these with MIC ≥32μg/ml, only 2 isolates of C. albicans were sensitive dose dependent.
Resistance to 5-flucytosine (MIC ≥4 μg/ml) was observed in 6 (14.6%) C. albicans isolates, 6 (33.3%) C. tropicalis isolates, 5 (14.7%) C. parapsilosis isolates (these were also resistant to fluconazole), 1 (20%) C. glabrata isolates and all 11 (100%) C. krusei isolates (these were also resistant to fluconazole).
Except 1 isolate of C. krusei which was resistant to voriconazole and showed MIC 2 μg/ ml, this one was also resistant to 5- flucytosine and fluconazole but sensitive to amphotericin B. All other Candida isolates were sensitive to voriconazole, many of these isolates showed high resistance to fluconazole ranging from 23.5% to 100%.
Discussion
Our study shows that Candida blood stream infections constitute a large percentage (15.8%) of all neonatal bloodstream infections our isolation of Candida is higher than some of recent studies [11-13] this could be due to the use of automated instrument and better isolation rates provided by the patented culture bottles, whereas most of other studies used conventional methods. In our study the Candida infection especially so by non albicans species was more common in VLBW (22) and LBW (34) neonates which have been reported as a risk factor in some recent studies [12-15]. We found its association highly significant and as the incidence of LBW and VLBW births increase along with their hospitalizations and increased survival for various reasons, the incidence of Candida sepsis is also increasing. The Change in pattern towards Candida infections has been partly attributed to increased immune suppression, premature birth, prolonged hospitalization and prior use of antimicrobials [10-18]. The genus Candida encompasses more than 150 species, only a few of which cause disease in humans [15]. With rare exceptions, the human pathogens are Candidaalbicans, Candida guilliermondii, Candida krusei, Candida parapsilosis, Candida tropicalis, Candida kefyr, Candida lusitaniae, Candida dubliniensis, and Candida glabrata. Overall, C. albicans typically has remained the leading Fungal pathogen but incidence of non-albicans species is increasing worldwide and constitute more than 60% of infections [15]; this is highlighted by our study also where high incidence of NAC (62.4%) is observed especially that of C. parapsilosis (31.2%) which could anytime surpass the incidence of C. albicans species if this trend continues. This species has been found to be more common in tropical countries as compared to western countries where its incidence is quite low [15], this fact is reconfirmed by our study. One of earlier studies in India reported C. grabrata as most common and C. parapsilosis as second most common agent of neonatal sepsis [2].
We found that the species identification performed by VITEK2 YST card by the instrument was mostly in agreement with conventional standard mycology techniques, with correct identification in 106 (97.2%) isolates, this observation was similar to a recent study [7].
The antifungal susceptibility data obtained in our study are similar to those of other studies published in recent years; the earlier studies reported 83% of C. tropicalis, 100% C. glabrata and up to 62.5% of C. albicans to be resistant to fluconazole [14,17,19,20]. This is in agreement with recent studies since a very high number of Candida isolates are resistant to fluconazole and more so for the NAC as seen in some recent studies [13,14,17,19,20]. Amongst NAC C. parapsilosis showed least resistance to fluconazole which has also been pointed out in a recent study [20], this aspect could be helpful in instituting appropriate antifungal therapy if timely identification of infecting isolate is made.
Amphotericin B resistance is not a cause of concern as far as C. albicans is concerned as seen in similar recent studies [13,14,17,19,20]. But it might be an issue if the infecting species is NAC such as C. parapsilosis which is known to have a high resistance and seems to be an increasing problem in neonates [15, 20]. Isolation of various candida species in India from past studies has been shown in [Table/Fig-6].
Isolation rates of various Candida species from bloodstream infection in earlier studies.
| Current study | Baradkar VP et al., [2] | Rani R et al., [12] | Wadile RG et al., [18] | Chander J et al., (Adult and neonates combined) [14] | Oberoi JK et al., (Adult and neonates combined) [20] |
|---|
| Sample size | 658 | 264 | 454 | 108 | 4651 | 69,010 |
| Bacterial isolates | 57.9% | NA | 20.73% | 38.8% | 0.94% | NA |
| Candida isolates | 15.8% | 19.14% | 11.01% | 18.5% | 0.05% | 1.74% |
| % of NAC (Non-albicans candida) isolates | 62.4% | 77.3% | 96% | 65% | 70.4% | 83.2% (2006-08) |
Many isolates especially those of C. krusei were resistant to 5 Flucytocine, one recent study has also shown similar findings in C. utilis and C. pelliculosa isolates [20]. Among all the isolates in our study voriconazole resistance was seen only in one isolate, however very few studies have performed 5 Flucytocine or voriconazole susceptibility testing. The ones who have performed these including our study indicate a low resistance for voriconazole in present situation [20], especially for NAC species where it could be used as a therapeutic tool in place of other drugs such as flucanozole and amphotecicin B.
Limitation
We could not reconfirm the isolates by molecular methods due to limited resources available at our institute.
In current study we could test MICs of only 4 drugs which were available on VITEK2 cards, further and more elaborate studies from various areas could throw more light into susceptibility trends for newer antifungal drugs like posaconazole and caspofungin.
Conclusion
From our study, it is clear that automated methods like VITEK2 and BACT/ALERT 3D is a useful tool and could provide better yield in lesser time as compared to conventional methods, although conventional methods remain the gold standard. Routine screening of Candida isolates to the species level and susceptibility confirmation in a properly equipped laboratory is very essential. This aspect has been highlighted by fact that NAC are increasing and all C. krusei isolates in our study were resistant to fluconazole and 5 flucytocine. Drugs like fluconazole can no longer be used without knowledge of the infecting species in suspected yeast bloodstream infections unless the strain is found to be sensitive. Hence antifungal resistance appears to be continuously evolving and for now voriconazole could be considered as a useful alternative in such cases.