Psoriasis is a chronic disease in which there is an acceleration of the normal growth cycle of the skin due to the activation of T-cells; subsequently, overproduction of arachidonic acid that leads to generation of various pro-inflammatory mediators like PGs (prostaglandins), LTs (leukotrienes), cytokines and adhesion molecules [1-3].
Secondly, it is not known when symptoms associated with MGD develop during the disease process or at what point of time, tear film tests derange after commencement of dry eye symptoms [8].
Omega-3 fatty acids (O3FAs) like Eicosapentaenoic Acid (EPA) exert an anti-inflammatory effect by modulating eicosanoid metabolism [9]. Potential benefit of O3FAs in inflammatory skin disorders has been documented recently; a prospective, observational study found that supplementary treatment with oral O3FAs (640mg/day) complements topical treatment in psoriasis, and makes a significant contribution to reducing Psoriasis Area and Severity Index (PASI), Nail Psoriasis Severity Index (NAPSI) and Dermatological Life Quality Index (DLQI) [10]. In a double-blind, randomized trial, intravenous administration of O3FAs significantly improved inflammatory skin lesions in chronic plaque-type psoriasis [11]. In another randomized controlled trial, dietary consumption of O3FAs significantly improved dry eye signs and symptoms in patients with rosacea [12]. In addition, O3FAs have also been found to be effective in the treatment of acne vulgaris [13].
However, conventional dry eye treatments like tear substitutes are temporary fixes as they do not alter dry eye pathophysiology (ocular surface inflammation) [14].
In the skin, resolving E1 has been found to inhibit dendritic cell migration and attenuation of contact hypersensitivity responses probably through LTB4-BLT1 signalling blockade [15]. Although the role of O3FAs in patients with MGD is relatively well established both experimentally and clinically, it is currently not known whether O3FAs are effective in psoriasis per se or skin and dry eye disease without MGD. A search of major databases (Medline, Scopus, Google Scholar and Embase) including the keywords ‘ocular psoriasis’ and ‘omega 3 fatty acids’ revealed that no trial has been done to determine this.
Thus, the aim of the present study was to investigate whether oral consumption of O3FAs are effective in psoriasis per se or were they effective only on the meibomian gland dysfunction which is often seen in these patients presenting with dry eye symptoms.
Materials and Methods
An interventional, non-randomized, controlled study was performed at School of Medical Sciences and Research, Sharda University, Greater Noida (centre 1), Rotary Eye Hospital, Palampur, India (center 2) and Laser Eye Clinic, Noida (center 3), referral eye centers in northern part of the Indian sub-continent from January 2014 to March 2015. The trial was approved by the institutional review boards and the local ethics committee and the study conformed to the Transparent Reporting of Evaluations with Non-randomized Designs (TREND) statement [16]. A written informed consent was obtained from all participating patients and the study followed the tenets of the declaration of Helsinki.
Inclusion Criteria
A single dermatologist (SS) made the diagnosis of the cutaneous disease and disease severity was assessed by the Psoriasis Area and Severity Index (PASI) [17]. Patients with psoriasis (chronic plaque/erythrodermic/pustular types) referred from dermatology or rheumatology clinics with dry eye symptoms or complaining of ocular irritation were invited to take part in the trial. The patients were first examined by an independent investigator, who was not a study surgeon, and the presence of ocular complaints was noted. The subjects were included in the study when they had dry eye symptoms and/or TBUT less than 10 seconds or Schirmer less than 6mm. The extent of damage to the ocular surface at cellular level, was assessed by Conjunctival Impression Cytology (CIC). Psoriatic patients, without dry eye symptoms and any clinical evidence of MGD {Dry Eye Scoring System (DESS) score less than three, absence of lid margin telangiectasias and easily expressible meibomian glands with translucent serous secretion} served as controls. They were well matched with respect to age, sex, and demography.
Symptomatic subjects were allocated to MGD and non-MGD groups, based on presence or absence of MGD; meibomian gland status was evaluated based on recommendations of the International workshop on meibomian gland dysfunction [18]. Dry eye symptoms were evaluated based on response of subjects to a dry eye questionnaire (Dry Eye Scoring System, DESS ©) [Table/Fig-1]. DESS has been recently validated in a multicenter study done at our center; it had a high level of internal consistency, as determined by a Cronbach’s alpha of 0.823. DESS is an 18 points questionnaire which characterizes dry eye patients based on severity (symptom free, mild, moderate, and severe). The minimum score for inclusion was three [19-21]. All patients were further questioned for disease duration, joint involvement, and treatment modalities received for the cutaneous disease.
Dry eye questionnaire and scoring system (DESS©).
| Symptom | Score (maximum 18) |
|---|
| Absent(0) | Sometimespresent (1) | Frequentlypresent (2) | Alwayspresent (3) |
|---|
| Itching or burning | | | | |
| Sandy or gritty sensation | | | | |
| Redness | | | | |
| Blurring of vision | | | | |
| Ocular fatigue | | | | |
| Excessive blinking | | | | |
*Scores of 0 to 6 were mild, 6.1 to 12 were moderate, and 12.1 to 18 indicated severely symptomatic dry eye. 23-26 ©Bhargava R. Laser Eye Clinic, Noida, India.
Exclusion Criteria
Patients with current ocular infection, psoriatic uveitis, history of Laser In Situ Keratomileusis (LASIK), allergic conjunctivitis, contact lens wearer, herpetic eye disease, and diabetes, were excluded. Patients with inability to swallow soft gel capsules, on aspirin or anti-coagulant therapy, and those allergic to fluorescein were also excluded from the study.
Sample Size Calculation
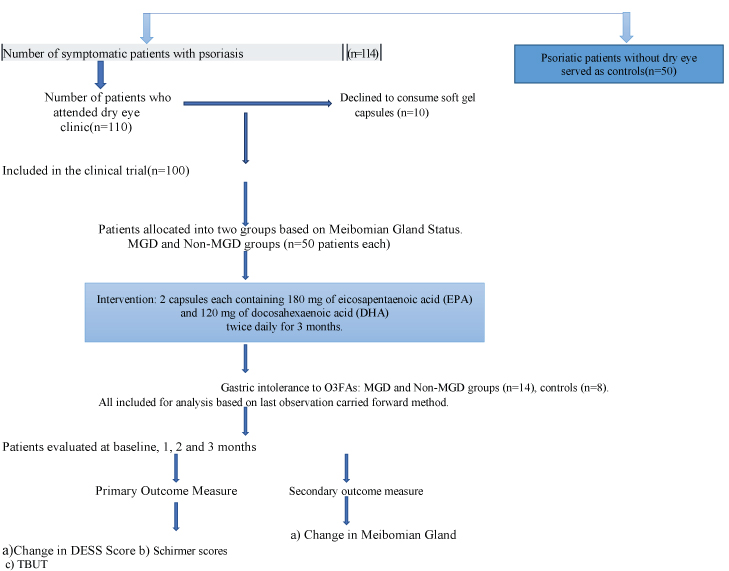
Sample size calculation was done based on the web calculator of the university of British Columbia [22]. This involved the principal of comparing means of two independent samples using one of the study variables. In the present study, the variable was dry eye symptoms (DESS score). So, a pilot study was first done on 15 subjects. The mean decrease in DESS score in MGD group was 0.73 and in without-MGD group was 0.61, respectively. The common standard deviation was 0.23. Assuming 1:1 allocation, 90% power (alpha=0.05) and a precision error of 5% to detect difference of 20% or more in DESS score between two groups, the estimated sample size in each group was calculated to be 52. [Table/Fig-2] shows the patient flowchart, allocation schedule and follow up protocol.
Flow-Chart showing enrolment, intervention, follow-up and analysis.
Instruction
All participants were advised to continue topical medications for psoriasis as advised by the dermatologist including vitamin D analogues, salicylic acid, and coal tar. However, systemic corticosteroids or topical medications (other than artificial tear supplements) that could directly affect tear film or meibomian gland function (retinoids, cyclosporine, beta-blockers, benzodiazepines, and anti-histamines) were discontinued four weeks prior to intervention. During this period, all participants were prescribed 0.5% carboxymethylcellulose eye drops four times a day. However, patients were instructed not to use tear supplements for at least two hours prior to tear film testing. Subjects were instructed not to work on computers for more than three hours per day [20]. The capsules were dispensed by healthcare personnel who were not involved in patient care. All subjects (MGD, Non-MGD and control groups) received two soft gel capsules, each containing 180 mg of Eicosapentaenoic Acid (EPA) and 120mg docosahexaenoic acid (DHA), twice daily for three months (total 720 mg of EPA +480 mg DHA/day). They were instructed to return the empty bottles of study capsules at monthly visit, and any unused capsules (if any) were counted to determine patient compliance with the study protocol wherein another pack with 120 capsules each were provided to them. The subjects were instructed to take a normal diet, and not to take any other additional dietary supplement.
Outcome Measures
Subjects were evaluated at baseline, one, two, and three months after the start of dietary supplementation. The primary outcome measure was decreased from baseline in subjective dry eye symptoms, three months after dietary supplementation was begun. Dry eye related symptoms like ocular fatigue, blurring of vision, itching, or burning, sandy or gritty sensation and redness respectively were assigned a score of 0-3 (DESS ©), when absent (0), sometimes present (1), frequently present (2), and always present (3). A DESS score of 0-6 was mild, 6.1-12 moderate and 12.1-18, severely symptomatic dry eye [Table/Fig-1].
The secondary outcome measures (three months after start of dietary supplementation) were changed in Meibomian Gland Score (MGS), Schirmer test value (tear production), TBUT (measure of tear film stability) and Conjunctival Impression Cytology (CIC).
Ophthalmic Examination and Measurements
The participants were instructed to visit the dry eye clinic in the morning. The independent investigator performed tear film tests at the same time of the day in a dimly lit room (between 10am and 12 pm). The independent investigator was not aware of the MGD status of the subjects. Subjects underwent Schirmer and TBUT and self-administered the DESS at all the visits. The independent investigator was masked to the information obtained from the questionnaire.
TBUT was first performed followed 30 minutes later by Schirmer test with anaesthesia (0.4% oxybuprocaine hydrochloride), as per the technique described in previous study. A TBUT of less than 10 seconds and length of wetting less than 6 mm was considered consistent with dry eye, respectively [23]. CIC was done as per the technique described Previously [24]. A light microscope with 100X low-power field was first used to localize the cells (10X objective). Then, the cells were analysed with 400X final magnification (40X objective). Atleast 10 High Power Field (HPFs) were examined to estimate goblet cell density (GCD) and epithelial cell morphology. Estimated GCD was the number of goblet cells counted per HPF divided by the sampling area covered in square millimetres. Grading was done by the criteria suggested by Nelson et al., Nelson grades 0 and 1 was normal Cytology [25], whereas grades 2 and 3 was abnormal cytology CIC.
Statistical Analysis
Statistical analysis was performed on an intent-to-treat basis using IBM, Statistical Package for the Social Sciences (SPSS) Statistics version 22.0 (IBM Corp., New York, NY). A one-way repeated-measures ANOVA was conducted to determine the significant changes in the mean test values over the course of three months of intervention (MGD, Non-MGD and control groups). There were no outliers and the data were normally distributed, as assessed by box plot and Shapiro-Wilk test (p>0.05), respectively. The assumption of sphericity was violated, as assessed by Mauchly’s test of sphericity. Therefore, a Greenhouse–Geisser correction was applied. Post-hoc test was done using Bonferroni correction to determine the level of significance by time; the F-statistic was reported as F (df time, df error) = F-value, p = p-value. A p-value<0.005 was considered statistically significant.
Results
Fifty patients each in MGD and non-MGD groups were compared with fifty age and sex matched controls. Environmental exposure of the participants did not differ significantly as most were urban, and were having similar work conditions.
The mean age (±SD) of subjects in MGD, non MGD and control groups was 46.2±4.2 (range, 32–74 years), 48.4±4.5 (range, 34–68 years) and 47.6±3.8 (range, 30-70 years), respectively; it did not differ significantly amongst the three groups (ANOVA, p=0.268). There were 21 men and 29 women in the MGD group and 27 men and 23 women in the non-MGD group. In controls, there were 24 men and 26 females, respectively. (ANOVA, P=0.834).
The mean duration of skin disease was 7.4±6.2 years (range 1–16 years) and 7.2±5.8 years (range 1-14 years) in MGD and non MGD groups, respectively. The PASI scores did not differ significantly between the three groups (ANOVA, p=0.132). [Table/Fig-3] shows the characteristics of subjects at baseline.
Characteristics of patients at baseline.
| Parameter | MGD | Non-MGD group | Controls | p-value (ANOVA) |
|---|
| Age (years) | 46.2±4.2 | 48.4±4.5 | 47.6±3.8 | 0.268 |
| Baseline DESS | 9±2.4 | 8.8±1.7 | 0.6 | 0.146 |
| Baseline Schirmer (mm) | 11.3±3.3 | 6.4±3.3 | 12±2.4 | 0.001 |
| Baseline TBUT (seconds) | 7±1.9 | 11.7±3.1 | 12.6±4 | 0.001 |
| Nelson Grade | 2.1±0.7 | 2.2±0.6 | 0.6±0.2 | 0.001 |
| Baseline MGS | 1.2±0.9 | 0.3 | 0.2 | 0.001 |
| Baseline PASI Score | 5.6 ± 2.8 | 5.46±3.2 | 5.3±3.4 | 0.132* |
*MGD (Meibomian Gland Dysfunction), TBUT (Tear film break up time), MGS (Meibomian gland Score), ANOVA (Analysis of covariance), NA (not applicable). Psoriasis Area and Severity Index (PASI), * t-test.
No patient had facial or eyelid psoriasis plaques. Gastric intolerance to O3FAs was the reason for dropout of six patients in MGD group and eight patients each in non-MGD and control groups, respectively. All dropouts were included for analysis based on the last observation carried forward method.
Dry Eye Symptoms
In MGD and non-MGD groups, repeated measure ANOVA revealed that O3FA dietary intervention elicited a significant change in dry eye symptoms over time (F (1.56, 123.12) =237.48, P<0.001) and {F (1.51, 3.16) =91.6, P<0.001}, respectively. Post-hoc analysis revealed that symptoms significantly decreased from pre-intervention to one month, two months, three months, and from two months to post-intervention in MGD group. In non-MGD group, symptoms significantly decreased, but only at three months.
Meibomian Gland Score
In MGD group, repeated measure ANOVA revealed that dietary intervention elicited a significant change in MGS over time (F (1.62, 128.2) =51.2, p<0.001). Post-hoc analysis revealed that this improvement was significant (p<0.001) at two months and three months post-intervention.
Tear Film Breakup Time
Tear film breakup time was abnormal (<10 s) in 88% and 22% patients in MGD and non MGD groups, respectively. In MGD group, repeated measure ANOVA revealed that dietary intervention elicited a significant change in TBUT over time (F(2.14, 246) =155.2, p<0.001). Post hoc analysis revealed that TBUT score significantly improved from baseline to one month, two months, three months, and from two months to post-intervention. In non-MGD group, there was a significant change in TBUT over three months of intervention (F (1.54, 17) =15, p<0.001). On post- hoc analysis, increase in TBUT scores from pre-intervention to one month and two months was not significant (p=0.499 and 0.269), respectively. However, TBUT scores increased significantly (p<0.001) at three months and from two months to three months. The change was not significant among controls (F (1.8, 109) =4.4, p=0.390).
Schirmer test
Schirmer was abnormal (wetting <6 mm) in 18% and 28% patients in MGD and non-MGD groups respectively at baseline. In MGD group, repeated measure ANOVA revealed that dietary intervention elicited a significant change in Schirmer scores over three months (F (1.68, 30.4)=44.23, p<0.001). On post-hoc analysis, increase in Schirmer scores from pre-intervention to one month (p=0.952) and two months (0.169) was not significant. However, Schirmer scores increased significantly (p<0.001) at three months and from two months to three months. In non-MGD group, Schirmer score increased significantly at three months (F (1.35, 21.34) =15.41, p<0.001). On post-hoc analysis, increase in Schirmer scores from baseline to one month and two months was not significant (p=0.904 and 0.499), respectively. However, Schirmer scores increased significantly (p<0.001) at three months and from two months to three months. The change was not significant among controls (F (1.6, 99) =2.1, p=0.130).
Nelson Grade
Repeated measures ANOVA revealed that there was a significant improvement in Nelson grade in MGD and Non-MGD groups, respectively (p<0.001). However, on Post-hoc analysis, this change was significant after three months of intervention in both the groups. The change in Nelson grade was not significant amongst controls (p=0.645).
[Table/Fig-4] shows the mean test values on repeated measure ANOVA in MGD, non-MGD and control groups, respectively.
Mean test values in meibomian gland (MGD), Non-MGD, and Control groups on repeated measure analysis of variance (ANOVA). Goblet Cell Density (GCD).
| MGD Group (n=50) | Non-MGD Group (n=50) | Control Group (n=50) |
|---|
| Parameter | BL | 1M | 2M | 3M | ANOVA | BL | 1M | 2M | 3M | ANOVA | BL | 1M | 2M | 3M | ANOVA |
|---|
| DESS score | 9±2.4 | 8±2 | 5.4±1.8 | 2.6±2.8 | p<0.001 | 8.8±1.7 | 8.7±1.7 | 6.2±2 | 3.5±2.4 | p<0.001 | 0.6 | 0.6 | 0.46 | 0.4 | p=0.876 |
| Schirmer (mm) | 11.3±3.6 | 11.3±3.5 | 11.4±3.6 | 12±3.4 | p<0.001 | 6.4±3.3 | 7.3±3.3 | 8.8±3.1 | 11.6±3.2 | p<0.001 | 12.6±1.8 | 12.6±1.8 | 12.9±1.7 | 13±1.7 | p=0.130 |
| TBUT (sec) | 7±1.9 | 9±2.6 | 10.2±3.1 | 11.6±2.8 | p<0.001 | 11.7±3.1 | 11.7±3 | 11.8±3.1 | 12±3 | p=0.124 | 12.9±1.6 | 13±1.6 | 13.1±1.5 | 13.2±1.5 | p=0.390 |
| MGS | 1.2±0.9 | 1.1±0.9 | 0.7±0.6 | 0.4±0.4 | p<0.001 | 0.3 | 0.3 | 0.26 | 0.2 | p=0.846 | 0.2 | 0.2 | 0.18 | 0.16 | p=0.945 |
| Nelson grade | 2.1±0.7 | 2±0.6 | 1.8±0.8 | 1.4±0.6 | p<0.001 | 2.2±0.6 | 2.2±0.6 | 1.8±0.6 | 1.5±0.6 | p<0.001 | 0.6±0.2 | 0.6±0.2 | 0.56±0.3 | 0.52±0.2 | p=0.645 |
| GCD (cells/mm2) | 392±156 | 410±166 | 476±138 | 510±146 | p<0.001 | 328±98 | 336±106 | 410±116 | 476±124 | p<0.001 | 756±178 | 764±184 | 770±212 | 776±184 | p=0.246 |
BL: Baseline, M1: Model 1, M2: Model 2, M3: Model 3
Discussion
This study investigated whether oral consumption of O3FAs are effective in psoriasis per se or are only effective on the MGD often seen in psoriatic patients with dry eye. Oral supplementation with O3FAs improved signs and symptoms of dry eye in both the groups, over a three month period in this clinical trial.
Symptomatic psoriatic patients with MGD had an abnormal TBUT despite a normal Schirmer score at baseline; repeated measure ANOVA suggested that improvement in DESS (symptoms) and tear film stability was prompt and at all time points in these patients (at one, two and three months of intervention).
On the contrary, patients without MGD had an abnormal Schirmer, abnormal Nelson grade but anormal TBUT at baseline; although there was a significant improvement in both Nelson grade and TBUT, but these parameters improved only after three months of intervention. Thus, the response may vary depending upon the underlying condition and duration of therapy, apart from an optimal dose. We hypothesize that this differential response was possibly due to different pathologic mechanisms underlying the dry eye disease in these patients. Dry eye disease in patients without MGD could probably stem from damage to the ocular surface or the lacrimal gland due to the psoriatic disease process (as evidenced by abnormal Nelson Grade) leading to aqueous tear deficiency, tears hyperosmolarity and ocular surface inflammation. The study also revealed that although there was a significant increase in Schirmer test values at study end-point in both groups; the increase in tear production was seen after two months in the MGD group but only after three months of intervention in the non-MGD group. On the contrary, tear film stability (TBUT) increased significantly at all time points in MGD group in contrast to that at three months in non-MGD group. This suggests that resolution of ocular surface damage/inflammation and consequent increase in tear production requires a longer therapy (in range of three months) in patients without meibomian gland dysfunction. In a double-masked randomized controlled study by Mayser et al., 75 subjects with chronic plaque psoriasis subjects were randomized to a 14-day treatment with either intravenous O3FAs or O6FAs emulsion. A manifold increase in plasma-free EPA concentration, neutrophil leukotriene B5 and platelet thromboxane B3 generation and reduction PASI scores was observed in the O3FA group [26]. O3FAs may be effective in limiting the spreading of the inflammatory process in the skin where the main route for the synthesis of leukotrienes is 15-lipoxygenase, giving rise to 15-hydroxyeicosatetraenoic acid. The epidermis can convert the leukotriene A4 (produced by the polymorphonuclear leukocytes) into leukotriene B4, one of the main inflammation mediators. Hydroxylated metabolites through 15-lipoxygenase, 15-hydroxyeicosapentaenoic acid, and 15-hydroxy docosahexaenoic acid are released following dietary consumption of EPA and DHA. Both these substances inhibit the 5-lipoxygenase of mononuclear cells, decreasing the synthesis of pro-inflammatory leukotrienes LTB4, LTC4, and LTD4 [10,27]. In another randomized controlled trial in ocular rosacea patients (n=130), dietary consumption of O3FAs for six months elicited a significant change in dry eye symptoms at all time points whereas, change in Schirmer test values was significant only after three months of intervention 11. This study highlighted the potential role of O3FAs in alleviating dry eye symptoms, skin, and ocular surface inflammation, in addition to MGD.
Limitation
The present study had certain limitations; first, the study design was a non-randomized. Secondly, adequate blinding could not be achieved (same treatment protocol for MGD, non-MGD and control groups, respectively), potentially confounded the results. Third, newer measures of ocular surface health like measurement of tear film osmolarity and markers like omega 3 index were not evaluated due to cost constraints.
Conclusion
The results of the present study highlighted the potential benefit of dietary omega 3 fatty acids for dry eye in patients with psoriasis with and without meibomian gland dysfunction. However, the non-MGD group required a longer intervention period for improved results. However, more studies discussing this topic should be conducted for validation of results.
*MGD (Meibomian Gland Dysfunction), TBUT (Tear film break up time), MGS (Meibomian gland Score), ANOVA (Analysis of covariance), NA (not applicable). Psoriasis Area and Severity Index (PASI), * t-test.