Uterine Arteriovenous Malformation (AVM) is a rare disease and is usually an acquired disorder which can remain asymptomatic or cause life-threatening bleeding. Prompt diagnosis and management in the appropriate clinical setting is crucial. Imaging can diagnose and sometimes predict the possibility of future severe bleeds. Embolization is the mainstay of treatment, however should be performed using proper techniques of selective embolization avoiding physiological blood vessels supplying normal structures.
CT pelvic angiography, Digital subtraction angiography, Gelfoam embolization, Ultrasound
Case Report
A 32-year-old lady presented with an episode of heavy bleeding two days earlier, on a background of multiple episodes of mild bleeding during the last three weeks. She had delivered her third child by normal delivery two months earlier.
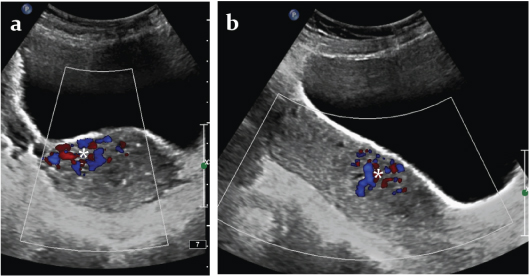
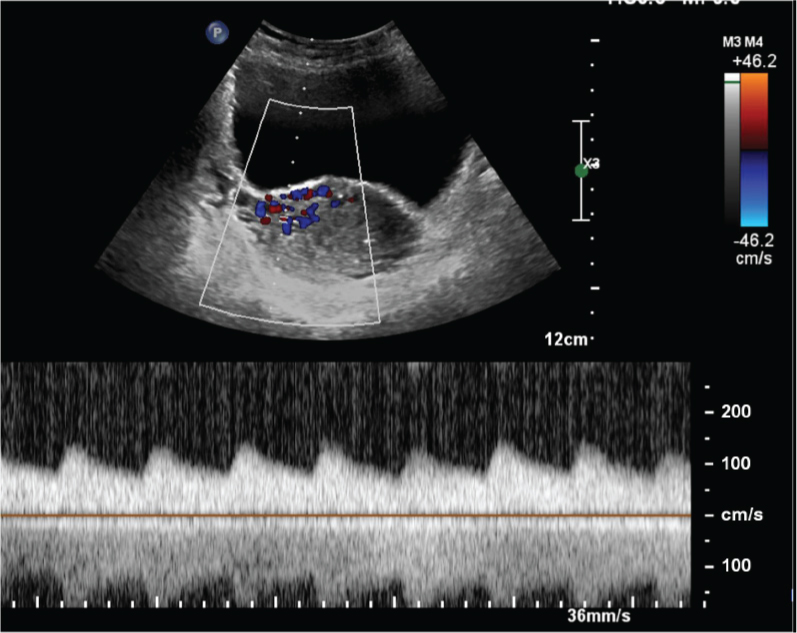
At presentation, she was stable with pulse rate of 96 beats per minute, blood pressure of 110/60 mm Hg and haemoglobin of 8.6 mg%. Transabdominal and transvaginal B mode ultrasound and colour Doppler showed a bulky uterus and an abnormal cluster of multidirectional colour flow in the anterior wall of the lower uterine corpus more towards the right side [Table/Fig-1a,b]. Spectral Doppler of the abnormal vessels showed low resistance high flow pattern [Table/Fig-2]. There were no retained products of conception.
Transabdominal- a) transverse and b) longitudinal ultrasonographic images of the uterus with colour Doppler showing a cluster of multidirectional colour flow (*) in the anterior wall of the lower uterine corpus more towards the right side.
Transabdominal transverse view of the uterus with colour and spectral Doppler showing a very low resistance high flow pattern.
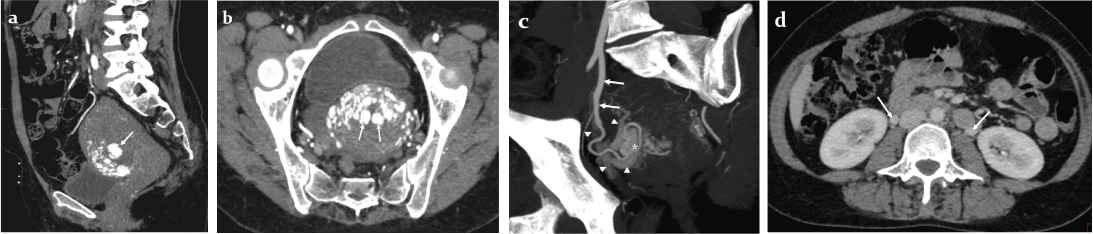
Raising a high suspicion of uterine AVM, a CT angiography of the pelvic vessels was performed which showed a tangle of blood vessels in the anterior wall of the lower uterine body representing the uterine AVM, early opacification of parauterine pelvic veins in the arterial phase, dilated bilateral ovarian veins, and most importantly, three large subendometrial vascular sacs representing either intranidal aneurysms or ectatic veins [Table/Fig-3a-d]. Beta hCG levels were within normal limits.
Contrast enhanced arterial phase computed tomography a) sagittal; b) oblique axial reformatted images of the uterus showing an area of hyperenhancement in the anterior wall of lower uterine corpus, and the subendometrial large vascular sacs (arrows); c) Double oblique maximum intensity projection arterial phase computed tomographic image showing an enlarged tortuous right uterine artery (arrows) supplying the AVM nidus (*) and partly opacified early draining veins (arrowheads); d) Axial CT venous phase image showing bilateral enlarged ovarian veins (arrows).
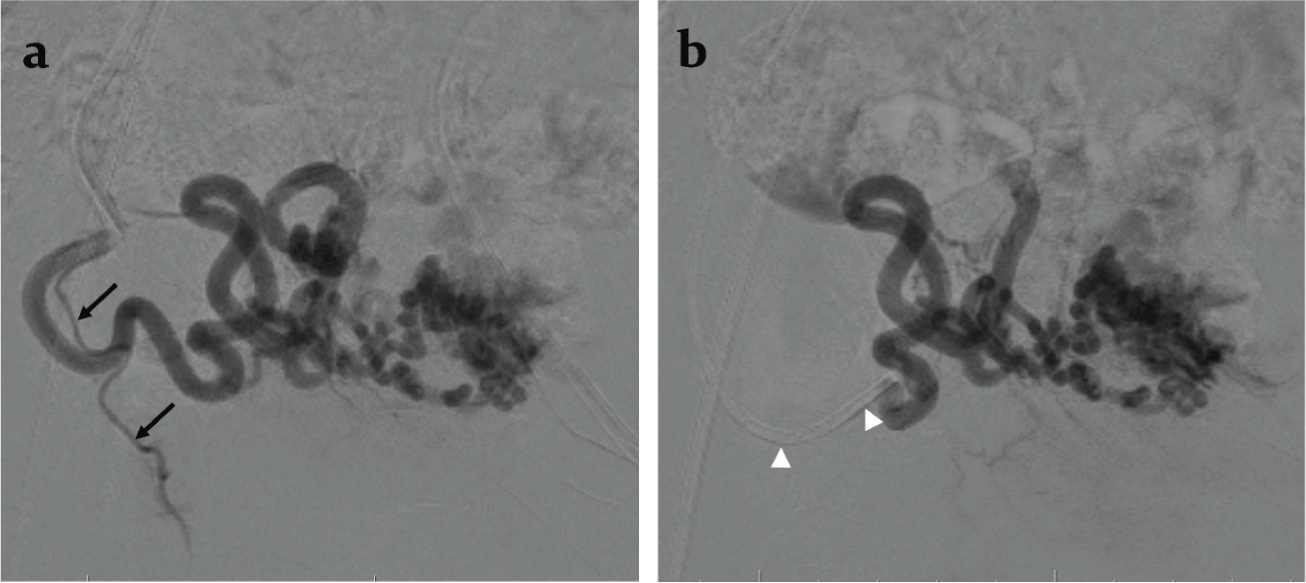
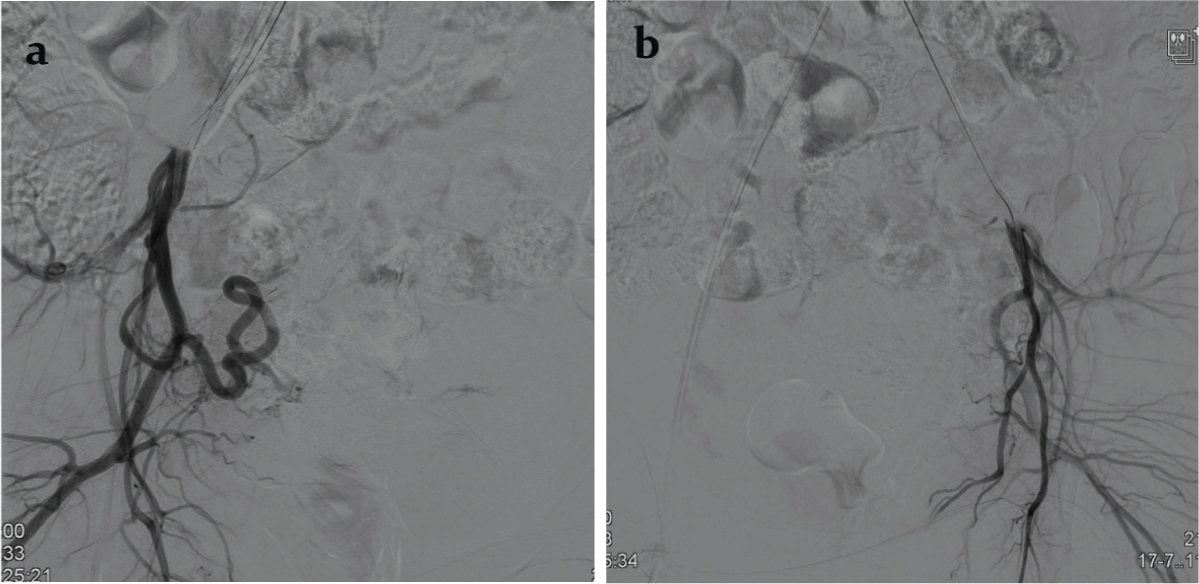
Because of history of a recent episode of heavy bleeding, borderline hemodynamic status and presence of predictors of heavy uterine bleed, decision to treat the AVM by uterine artery embolization was arrived at. Digital Subtraction Angiography (DSA) performed during uterine artery injections on both sides through right femoral access and using a Robertson uterine angiography catheter confirmed the AVM fed principally by the right uterine artery and showed venous aneurysms [Table/Fig-4a-c]. Since the flow rate within the AVM was not too rapid and there was no direct large arteriovenous shunt, gelfoam pledgets were chosen for embolization. After partial embolization of the AVM through the right uterine artery, an angiogram revealed a yet undemonstrated cervical artery [Table/Fig-5a]. The catheter was navigated further closer to the AVM past the origin of the cervical artery and embolization was completed [Table/Fig-5b]. The left uterine artery was seen to supply a hypertrophic vascular bed which was also embolized using gelfoam pledgets. Post embolization DSA of uterine artery on both sides showed complete occlusion of the AVM [Table/Fig-6a,b].
Digital subtraction angiography a) right uterine artery and (b and c) left uterine artery injections; c) being a venous phase image. Note enlarged tortuous uterine arteries (arrows) supplying a hypervascular bed, early draining venous channels (arrow heads) and venous aneurysm (*).
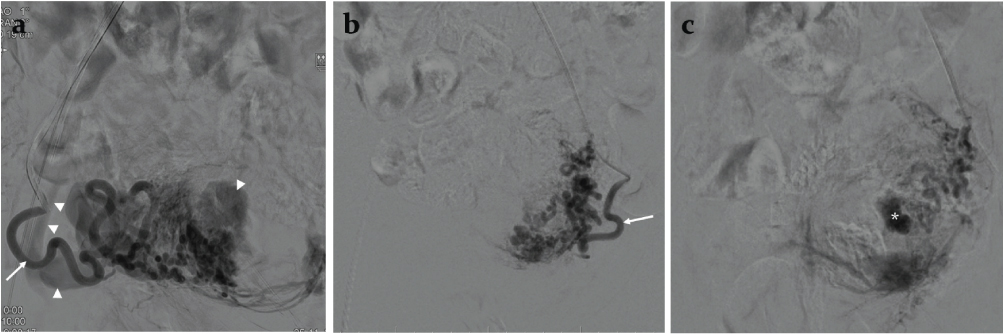
a) Intra-procedural digital subtraction angiography performed after partial embolization through the right uterine artery showing a cervical artery (arrows) arising from the uterine artery; b) Digital subtraction angiography after catheter (arrowheads) repositioning showing optimal position of the catheter in the right uterine artery past the origin of the cervical artery.
Post embolization a) right uterine artery and b) left uterine artery digital subtraction angiography showing occlusion of the AVM.
The patient had only two episodes of spotting after the procedure and was discharged the next day. The patient did not have any further episodes of bleeding and her normal menses resumed six weeks later.
Discussion
AVMs are abnormal non-physiological communications between arterial and venous system by passing the capillaries [1–9]. These can affect any part of the body and can occur in the uterus either as a congenital or acquired abnormality, acquired being more common than congenital [2,4,7]. It often presents with bleeding per vaginum, sometimes torrential and can prove to be fatal if not diagnosed early and managed appropriately.
Congenital AVM consist of multiple arteriovenous shunts and may involve extrauterine structures in the pelvis. Acquired AVM are far commoner and may occur following uterine trauma including iatrogenic ones such as curettage or caesarean section, uterine infection, retained products of conception, trophoblastic diseases, choriocarcinoma, endometrial or cervical carcinoma [1–3,5–9]. The most common presentation is abnormal bleeding per vaginum. Less common presentations include lower abdominal pain, dyspareunia, recurrent abortions and very rarely high output cardiac failure [3–6]. Early diagnosis is important not only to direct appropriate timely treatment, but also to avoid a curettage which can cause catastrophic bleeding [1].
Diagnosis is usually suggested by an ultrasound with colour Doppler which shows a focal cluster of abnormal multidirectional increased flow signal within the uterus [1,3–9]. Ultrasound also can diagnose associated or alternate diagnoses of retained products of conception, which is one of the most common causes of post pregnancy or post abortion bleeding per vaginum. Beta hCG should also be checked after a sonographic suspicion of AVM is raised to rule out the possibility of gestational trophoblastic disease, since a sonographic differentiation between the two is not usually possible [3]. A focal hypervascularity can be seen in post pregnancy or post abortive uterus at the implantation site secondary to normal physiological changes in the myometrial vascularity, which might mimic an AVM. This gradually regresses and disappears [10]. Presence of retained products of conception and gestational trophoblastic disease induces the persistence of this hypervascularity [11]. These pathologies however do not show an arteriovenous communication and early draining vein in CT or MR angiography studies.
For confirmation of diagnoses, usually an MRI or a CT pelvic angiography is performed, but the patient can be directly subjected to catheter angiography for diagnosis if an emergent indication for embolization exists. CT and MR angiography can confirm the diagnosis by showing an early draining vein, show clear extent of AVM, feeding vessels, presence of gestational trophoblastic disease and presence of predictors of severe bleeding like enlarged subendometrial vascular channels as in the present case [1,3–5]. Enlarged vascular sacs in an AVM represent either an intranidal aneurysm or venous ectasia. These indicate high flow, and when present within the endometrial cavity or in subendometrial location, predict a higher chance of severe bleed and requirement of an intervention.
Multiple treatment options exist and choice depends on the age, presentation, haemodynamic status, patient’s desire to retain uterus, desire for future conception and pregnancy, and the available expertise in the place of treatment [1–8,12,13]. Conservative management is followed when the AVM is incidentally detected or if the bleeding episodes are minimal, not persisting or decreasing. Otherwise, among the other options, embolization is the most widely performed treatment. Embolizing agents used include gelfoam, polyvinyl alcohol particles, cyanoacrylate glue and coils; choice depends on the speed of flow through the AVM, presence of prominent direct arteriovenous shunts, availability of materials and operator expertise [1–6,8,12,13]. Like any other embolization procedure, multiple intraprocedural check angiographies should be undertaken during uterine artery embolization, because some cervical or vaginal arteries or utero-ovarian anastomoses may become apparent after partial embolization of the pathological vessels, which if undetected would be unnecessarily embolized, sometimes resulting in cervical or vaginal necrosis or early ovarian failure.
The present case illustrates this necessary step of caution – initially undemonstrated cervical artery arising from the right uterine artery becoming apparent after partial embolization of the AVM. Untoward embolization of cervical or vaginal branches can be prevented by further distal placement of catheter, or usage of microcatheter placed further past the origin of these arteries. Fertility preservation post uterine artery embolization has always been a concern [1,3,7,9], even though there are multiple reports of successful pregnancy post embolization [7,12,13], Hysterectomy is considered the last option. There are descriptions in literature of a few other management options used successfully including oral contraceptives, hormonal intrauterine device, hysteroscopic and laparoscopic surgeries [1,9,14].
Conclusion
Uterine AVM is a rare disorder, and can sometimes cause severe and fatal bleeding. Early diagnosis and management is important in the right clinical setting. Ultrasound confirms the diagnosis in most cases and if patient is not stable for conservative management, embolization is the most preferred method of treatment. Apart from selective uterine artery cannulation, multiple intraprocedural angiographies should be performed to prevent any untoward cervical, vaginal or ovarian ischemia.
[1]. Selby ST, Haughey M, Uterine arteriovenous malformation with sudden heavy vaginal hemmorhageWest J Emerg Med 2013 14(5):411-14. [Google Scholar]
[2]. Hashim H, Nawawi O, Uterine arteriovenous malformationMalays J Med Sci 2013 20(2):76-80. [Google Scholar]
[3]. Alessandrino F, Di Silverio E, Moramarco LP, Uterine arteriovenous malformationJ Ultrasound 2013 16(1):41-44. [Google Scholar]
[4]. Karadag B, Erol O, Ozdemir O, Uysal A, Alparslan AS, Gurses C, Successful Treatment of Uterine Arteriovenous Malformation due to Uterine TraumaCase Rep Obstet Gynecol 2016 2016:01-03. [Google Scholar]
[5]. Bagga R, Verma P, Aggarwal N, Suri V, Bapuraj JR, Kalra N, Failed angiographic embolization in uterine arteriovenous malformation: a case report and review of the literatureMedscape J Med 2008 10(1):12 [Google Scholar]
[6]. Farias MS, Santi CC, Lima AAA, de A, Teixeira SM, De Biase TCG, Radiological findings of uterine arteriovenous malformation: a case report of an unusual and life-threatening cause of abnormal vaginal bleedingRadiol Bras 2014 47(2):122-24. [Google Scholar]
[7]. Vilos AG, Vilos GA, Hollett-Caines J, Rajakumar C, Garvin G, Kozak R, Uterine artery embolization for uterine arteriovenous malformation in five women desiring fertility: pregnancy outcomesHum Reprod 2015 30(7):1599-605. [Google Scholar]
[8]. Visvalingam G, Visvalingam G, Lee RWK, Tan TY, Tan HH, An unusual case of acquired uterine arteriovenous malformation with persistent scar ectopic pregnancy successfully managed with uterine artery embolizationJ Med Cases 2016 7(4):143-47. [Google Scholar]
[9]. Calzolari S, Cozzolino M, Castellacci E, Dubini V, Farruggia A, Sisti G, Hysteroscopic management of uterine arteriovenous malformationJSLS J Soc Laparoendosc Surg 2017 21(2):piii [Google Scholar]
[10]. Nayak S, Abnormal course of right renal artery and ovarian vessels: A Case ReportInternet J Biol Anthropol 2007 2(1):01-03. [Google Scholar]
[11]. Kido A, Togashi K, Koyama T, Ito H, Tatsumi K, Fujii S, Retained products of conception masquerading as acquired arteriovenous malformationJ Comput Assist Tomogr 2003 27(1):88-92. [Google Scholar]
[12]. Chia YN, Yap C, Tan BS, Pregnancy following embolisation of uterine arteriovenous malformation--a case reportAnn Acad Med Singapore 2003 32(5):658-60. [Google Scholar]
[13]. Amagada J, Karanjgaokar V, Wood A, Wiener J, Successful pregnancy following two uterine artery embolisation procedures for arteriovenous malformationJ Obstet Gynaecol (Lahore) 2004 24(1):86-87. [Google Scholar]
[14]. Chen S-Q, Jiang H-Y, Li J-B, Fan L, Liu M-J, Yao S-Z, Treatment of uterine arteriovenous malformation by myometrial lesion resection combined with artery occlusion under laparoscopy: a case report and literature reviewEur J Obstet Gynecol Reprod Biol 2013 169(2):172-76. [Google Scholar]