Adult Solid Hepatic Mesenchymal Hamartoma Masquerading as Malignancy
Gunjan Desai1, Prasad Pande2, Chandralekha Tampi3, Dattaprasanna Kulkarni4
1 Registrar, Department of Gastrointestinal Surgery, Lilavati Hospital and Research Centre, Mumbai, Maharashtra, India.
2 Registrar, Department of Gastrointestinal Surgery, Lilavati Hospital and Research Centre, Mumbai, Maharashtra, India.
3 Consultant, Department of Pathology, Lilavati Hospital and Research Centre, Mumbai, Maharashtra, India.
4 Consultant, Department of Gastrointestinal Surgery, Lilavati Hospital and Research Centre, Mumbai, Maharashtra, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Gunjan Desai, Lilavati Hospital and Research Centre, Mumbai-400050, Maharashtra, India.
E-mail: dsshlsh@gmail.com
Solid Hepatic Mesenchymal Hamartoma (HMH) rarely occurs in adults. We report two cases of solid adult HMH. A 62-year-old female with right upper abdominal pain on Computed Tomography (CT) scan revealed a well defined heterogeneously enhancing solid mass lesion in segment VII of liver along with non-enhancing central necrotic areas. Percutaneous biopsy and intraoperative frozen section were inconclusive and conventional right hepatectomy was done. Final histopathology was solid HMH.
Another 63-year-old female with right upper abdominal pain had cirrhotic liver, choledocholithiases, cholelithiases, mild ascites and a right lobe lesion with calcifications on CT scan. Ultrasound guided biopsy showed fibrocollagenous tissue. She was operated for a right hepatectomy and choledochoduodenostomy. Final histopathology revealed solid HMH. Though solid adult HMH is rare, it should be considered in differential diagnosis of solid/cystic/focal or multifocal liver lesions and surgical resection to negative margins is the treatment of choice.
Hamartoma liver, Liver cancer, Mesodermal masses
Case Report
Case 1
A 62-year-old lady presented with non-specific right upper abdominal pain without vomiting, jaundice, anorexia, fever, menstrual, urinary or bowel symptoms and no abdominal mass on palpation. All blood investigations including complete blood counts, liver function tests, renal function tests, amylase and lipase were normal.
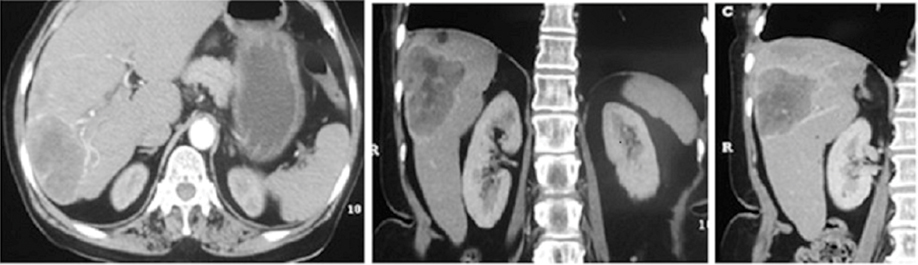
Ultrasonography revealed ill defined liver mass (size-5 cm) in segment VII. Contrast Enhanced Computed Tomography (CECT) scan revealed a well defined heterogeneous solid mass in segment VII of non-cirrhotic liver with a few non-enhancing central necrotic areas without calcification, central scar or fat containing areas [Table/Fig-1] Carcinoembryonic Antigen (CEA), CA-19.9, Lactate Dehydrogenase (LDH), CA-125, Human Chorionic Gonadotropin (HCG) and Alpha Feto Protein (AFP) were within normal limits [1-4].
CECT reveals a well defined heterogeneously enhancing solid mass lesion in posterior superior segment [segment VII] of right lobe of liver along with few non-enhancing central necrotic areas. No areas of calcification, central scar or fat containing areas seen. The liver otherwise appears non-cirrhotic.
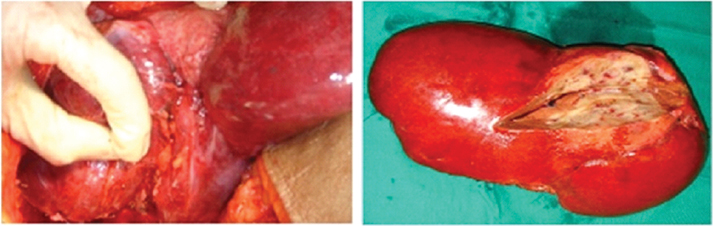
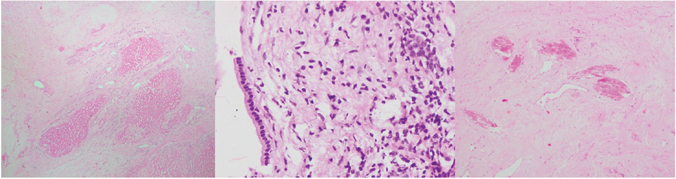
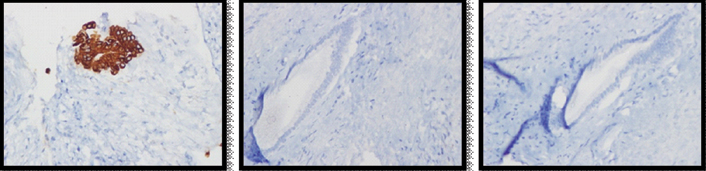
A percutaneous biopsy as well as intraoperative frozen section were inconclusive and showed only atypical cells. A conventional right hepatectomy was done [Table/Fig-2]. Final histopathology revealed a well circumscribed, encapsulated solid mass with central collagenous and fibrous core surrounded by few cystic spaces [Table/Fig-3]. Immunohistochemistry showed chromogranin, hepatocyte specific antigen negativity and CK7 positivity in ductal elements entrapped within the mass [Table/Fig-4]. The diagnosis was solid HMH. The patient recovered uneventfully, was discharged on eighth postoperative day, is doing well at follow up eight years later.
Intraoperative image showing the mass after liver mobilization on the left and specimen of right hepatectomy with cut section showing the solid encapsulated mass containing fleshy areas interspersed with few cystic areas in the lesion in the right image.
Histopathology images show scattered entrapped benign hepatocytes in abundant fibromyxoid stroma (left, H&E stain 40X), entrapped bile ducts with normal lining (middle, H&E stain 400X0 and dystrophic calcification in the stroma (right, H&E stain 100X).
Immunohistochemistry (400X images) done on the mass showed positivity for cytokeratin (left) and negativity for chromogranin (middle) and hepatocyte specific antigen (right).
Case 2
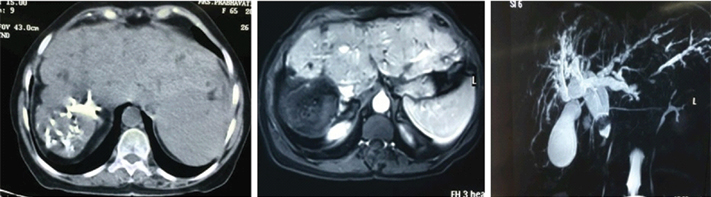
A 63-year-old female presented with episodic right upper abdominal pain since two years with increased intensity in last 20 days with loss of appetite, weight loss, icterus and mild ascites. Her haemoglobin was 9 mg/dl, direct bilirubin and alkaline phosphatase were elevated and hepatitis viral markers were negative. She was child pugh class A. Triphasic abdominal CT scan showed nodular cirrhotic liver with right lobe atrophy-left lobe hypertrophy complex, caudate hypertrophy with hypo attenuating 7.4 x 7.3 cm mass in right lobe with calcification [Table/Fig-5]. There was intrahepatic biliary radical dilatation, common bile duct dilatation, choledocholithiases and gall stones. Magnetic Resonance Imaging (MRI) showed hyperintense T2 and hypointense T1 lesion in right lobe liver with restricted diffusion and no enhancement in post-contrast images.
Triphasic abdominal CT scan shows nodular cirrhotic liver with right lobe atrophy-left lobe hypertrophy complex, caudate hypertrophy with hypoattenuating 7.4 x 7.3 cm mass in right lobe with calcification. Magnetic resonance imaging {middle image} shows lesion in right lobe liver with no enhancement in post-contrast images. Cholangiopancreaticogram {right image} shows intrahepatic biliary radical dilatation and common bile duct dilatation.
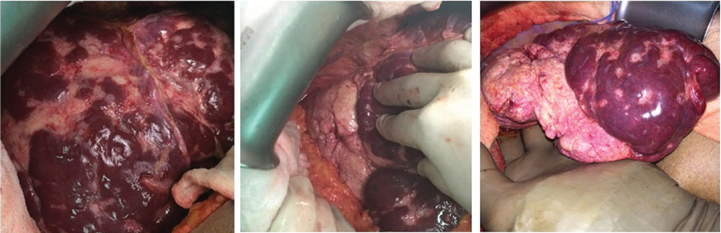
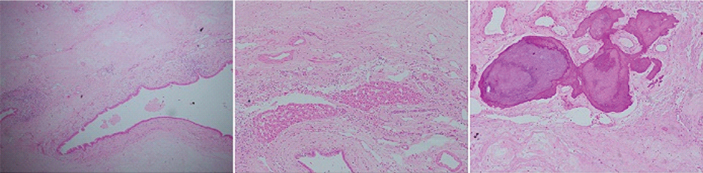
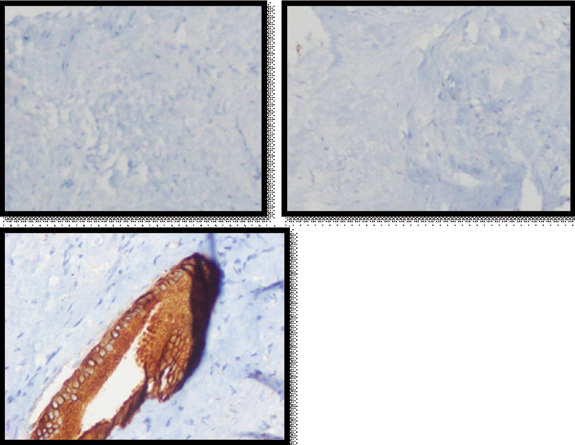
Ultrasound guided biopsy of the mass revealed fibrocolla-genous tissue. An anatomical right hepatectomy with choledochoduodenostomy was performed [Table/Fig-6]. The final histopathology revealed fibromyxoid stroma with prominent blood vessels, areas of calcification, scattered branching and dilated benign bile ductules with islands of entrapped hepatocytes and diagnosed solid HMH [Table/Fig-7]. Immunohistochemistry showed cytokeratin positivity and chromogranin and hepatocyte specific antigen negativity [Table/Fig-8]. Recovery was uneventful. The patient is doing well at one year follow up.
Intraoperative image shows cirrhotic and congested liver on extreme left image, the mass in its in vivo location in right lobe of liver in middle image and specimen after resection in the right image.
Histopathology images show scattered benign ductules in abundant fibromyxoid stroma (left), entrapped islands of unremarklable hepatocytes (middle) and dystrophic calcification in the stroma (right) (H&E stain 40X).
Immunohistochemistry images for the mass showing chromogranin (left, 100X) and hepatocyte specific antigen (middle. 100X) negativity and cytokeratin (right, 400X) positivity.
Discussion
HMH occurs rarely in adults [5]. It was considered a developmental ductal plate anomaly rather than a true cystic neoplasm [5]. Progressive enlargement is a result of fluid accumulation and cystic degeneration [1]. It usually presents with non-specific symptoms and is often misdiagnosed [6].
HMH was first reported by Edmondson in 1956 [7]. It is third most common liver mass in infants and children accounting for 22% benign liver lesions and is very rarely seen in adults [5,6]. 55% of cases present in infancy. Average age of presentation is 10-15 months. It can result in foetal hydrops, respiratory distress and intrauterine foetal death due to its large size [5]. They are more common in females in adults and in males in children [5,6]. They can be solid, cystic or mixed solid–cystic. Solid variant is most common in children where as cystic form is more common in adults [1,6].
Pathogenesis of HMH is not clear. One theory suggests that hepatocytes may be entrapped from surrounding hepatic lobules. Aberrant derivation of the tumour component by extension of primitive hepatic diverticulum growing into septum transversum is another theory. Recent developments suggest origin from hepatic stellate cells [2]. The cystic nature of HMH is the result of cystic degeneration of myxoid stroma or cystic dilatation of bile ducts. Rarely, solid form of HMH containing a larger amount of hepatocytes has been documented in adults [1,2,6]. Wiedemann syndrome, placental mesenchymal dysplasia and balanced translocation involving chromosome 19q13.4 have been reported [2,6].
Adult HMH might be premalignant and the evidence supporting this includes the rare cases of malignant transformation and presence of aneuploidy in flow cytometry which have shown a suspicious relationship between HMH and undifferentiated embryonal sarcoma. A case has been reported where angiosarcoma arose from an untreated HMH in a female patient [2,3].
It may be found incidentally during routine health check up or imaging or can present with abdominal pain, abdominal distention, upper abdominal mass or with inferior vena cava compression [6]. Spontaneous rupture of HMH causing biliary peritonitis has been reported [8]. Blood investigations and liver function tests are usually normal and tumour markers are not elevated as is also evident in our case.
On CECT, cystic HMH appears as large, non-calcified, low attenuation mass often with enhancing septa. However, our case, being a solid form, showed a heterogenously enhancing mass with no septations, which was an atypical finding. On MRI, cystic mesenchymal hamartomas may show low or high signal intensity lesions on T2 images. Solid hamartomas on the other end enhance brilliantly on post contrast delayed MRI images. They are non-18-Flurodeoxyglucose (FDG) avid on PET [9].
Differential diagnoses include hepatoblastoma, hepatocellular carcinoma, infantile haemangioendothelioma, biliary cystadenocarcinoma and undifferentiated embryonal sarcoma [6,9]. Percutaneous biopsy is often inconclusive as in our case and the treatment of choice is complete excision since a malignancy cannot be ruled out. Liver transplantation is an option where excision is not possible [6]. Both open or laparoscopic, anatomical or non-anatomical resections have been found to achieve cure. Recurrence has not been reported after complete excision [6,9].
Pathologically, the lesion is composed of abundant myxoid connective tissue interspersed blood vessels, enlarged bile ductules and entrapped hepatocytes. The adult HMH shows a variety of histological changes, the most common being cystic degeneration with loss of hepatocytes and bile duct epithelium presenting as a cystic lesion [1,5]. Immunohistochemistry shows vimentin and actin positivity in the stromal cells and cytokeratin 7 positivity of bile duct epithelial cells [5]. HMH has a good prognosis and long term survival is seen after a complete excision [1,5]. The differences between solid and cystic HMH are summarized in [Table/Fig-9] [1-4].
Differences between solid and cystic HMH [1-4].
| SOLID HMH | CYSTIC HMH |
|---|
| Demography |
| More common in infants children | More common in adults |
| More common in males | More common in females |
| Pathogenesis |
| Immature form of HMH with preserved hepatocytes and small bile ducts present | Mature ordinary HMH formed due to loss of hepatocytes, degeneration of biliary epithelium and cystic mesenchymal change |
| Pathology |
| Mass is tough and white on cut section | Cystic surface with clear or turbid pale yellow fluid |
| Tightly packed hepatic tissue with hyperplastic bile ducts and periductal fibrosis called ‘periductal collaring’ – Forms a fibroglandular mass | Mesenchymal tissue that is left separates the cystic areas. Cysts are lined by simple bile duct epithelium |
| Vascular proliferation is present and solid HMH has few blood vessels | There is no vascular proliferation and cystic HMH has no blood vessels |
| Bile ducts are small | Bile duct are tortuous or pseudocyst like |
| Tumour marker levels |
| Alpha feto protein can be significantly high | Alpha feto protein is usually normal |
| Radiology |
| On CECT, solid HMH, shows a heterogenously enhancing mass with or without septations | On CECT, cystic HMH appears as large, non-calcified, low attenuation mass often with enhancing septa. |
| On MRI, it is T1 hypointense and heterogeneously hyperintense on T2. It shows gradually increasing enhancement on gadolinium MRI with mild annular enhancement in arterial phase to brilliantly enhancing mass in delayed phase | On MRI, it is T1 hypointense to isointense and hyperintense on T2. It is nonenhancing or variably enhancing on gadolinium MRI as it has no blood vessels. |
| Management |
| Management is same for both the lesions and complete laparoscopic or open resection to negative margins or liver transplantation are the surgical treatment options |
Conclusion
Solid adult HMH is a rare tumor-like liver lesion with many imaging features similar to malignant liver lesions. It should be considered in differential diagnosis of enlarging solid/ cystic/focal or multifocal liver lesions in adults. Complete surgical resection is treatment of choice and is curative.
[1]. Chau K, Ho J, Wu P, Yuen W, Mesenchymal hamartoma of liver in a man: comparison with cases in infantsJournal of Clinical Pathology 1994 47(9):864-66. [Google Scholar]
[2]. Abrahao-Machado LF, de Macedo FC, Dalence C, Mesenchymal Hamartoma of the Liver in an Infant with Beckwith-Wiedemann Syndrome: A Rare Condition Mimicking HepatoblastomaACG Case Reports Journal 2015 2(4):258-60. [Google Scholar]
[3]. Tucker S, Cooper K, Brownschidle S, Wilcox R, Embryonal (Undifferentiated) sarcoma of the liver with peripheral angiosarcoma differentiation arising in a mesenchymal hamartoma in an adult patientInternational Journal of Surgical Pathology 2011 20(3):297-300. [Google Scholar]
[4]. Chang H, Jin S, Park C, Park Y, Jang J, Park C, Mesenchymal hamartomas of the liver: comparison of clinicopathologic features between cystic and solid formsJournal of Korean Medical Science 2006 21(1):63 [Google Scholar]
[5]. Klaassen Z, Paragi PR, Chamberlain RS, Adult Mesenchymal Hamartoma of the Liver: Case Report and Literature ReviewCase Reports in Gastroenterology 2010 4(1):84-92. [Google Scholar]
[6]. Pandey A, Gangopadhyay AN, Sharma SP, Long-term Follow up of Mesenchymal Hamartoma of Liver- Single Center StudySaudi Journal of Gastroenterology : Official Journal of the Saudi Gastroenterology Association 2011 17(1):20-22. [Google Scholar]
[7]. Edmondson HA, Differential diagnosis of tumors and tumor-like lesions of liver in infancy and childhoodAm J Dis Child 1956 91:168-86. [Google Scholar]
[8]. Batista-Castillo R, González-Martínez S, Jorba-Martin R, Memba-Ikuga R, Mata-Sancho F, González-Santín V, Biliary peritonitis secondary to spontaneous rupture of hepatic mesenchymal hamartomaRevista Española de Enfermedades Digestivas 2013 105(6):358-59. [Google Scholar]
[9]. Anil G, Fortier M, Low Y, Cystic hepatic mesenchymal hamartoma: the role of radiology in diagnosis and perioperative managementThe British Journal of Radiology 2011 84(1001):91-94. [Google Scholar]