Inguinal Hernia as an Initial Presentation of Pseudomyxoma Peritonei
Biswajit Dey1, Gourav Kaushal2, Prita Pradhan3, Pampa Ch Toi4, Biju Pottakkat5
1 Senior Resident, Department of Pathology, Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry, India.
2 Senior Resident, Department of Surgical Gastroenterology, Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry, India.
3 Junior Resident, Department of Pathology, Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry, India.
4 Associate Professor, Department of Pathology, Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry, India.
5 Professor, Department of Surgical Gastroenterology, Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Biswajit Dey, Senior Resident, Department of Pathology JIPMER, Puducherry-605006, India.
E-mail: drbish25@rediffmail.com
Pseudomyxoma Peritonei (PMP) is characterized by presence of abundant extracellular mucin in the peritoneum. Preoperative diagnosis of pseudomyxoma peritonei is difficult due to its varied presentation. We report a case of pseudomyxoma peritonei in a 64-year-old male, who initially presented with right inguinal hernia and then with recurrence as left inguino-scrotal hernia. A debulking surgery including subtotal colectomy, cholecystectomy and splenectomy along with peritonectomy was performed. The left inguino-scrotal hernia was reduced and a left inguinal hernioplasty was performed. This case is reported to highlight that pseudomyxoma peritonei can present as inguinal hernia and therefore, a thorough examination of the hernial contents for mucin should be done.
Mucin, Hernioplasty, Inguinoscrotal
Case Report
A 64-year-old male presented with abdominal discomfort, anorexia, post-cibal bloating and intermittent fever for three months. He had jaundice for one week. He did not complain of any abdominal pain, vomiting or gastrointestinal bleeding. He gave history of right inguinal hernia repair 10 years ago.
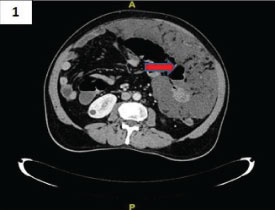
On physical examination, a lump was palpable in the left hypochondrium and periumbilical region. He had a healed right inguinal scar and a reducible left inguino-scrotal hernia. There was no tenderness or muscle guarding. On investigation, haemoglobin was 9.4 gm%, total leucocyte count was 7,200/cmm and platelet count of 4.2 lacs/cmm. On abdominal ultrasonography, massive fluid collection with high echoic spots were observed. Contrast Enhanced Computed Tomography (CECT) showed contracted omentum, which was pushed to left side of abdomen forming cocoon associated with ascites [Table/Fig-1]. There was evidence of cholelithiasis and a left inguino-scrotal hernia.
CECT showed contracted omentum, which was pushed to left side of abdomen (arrow) forming cocoon associated with ascites.
Laparotomy was performed and intraoperatively there was a loculated peritoneal mass adherent to the transverse colon associated with gelatinous ascites. Liver surface showed ‘scalloping effect’. Sigmoid colon loops was found inside the left inguino-scrotal hernia sac along with mucinous deposits. The loops were adherent to the hernia sac. However, the appendix could not be identified. Around 500 ml of mucinous material was aspirated out. A debulking surgery including subtotal colectomy, cholecystectomy and splenectomy along with peritonectomy was performed. The left inguino-scrotal hernia was reduced and a left inguinal hernioplasty was performed.
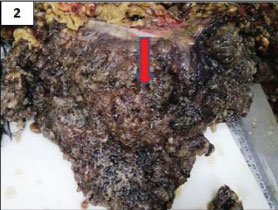
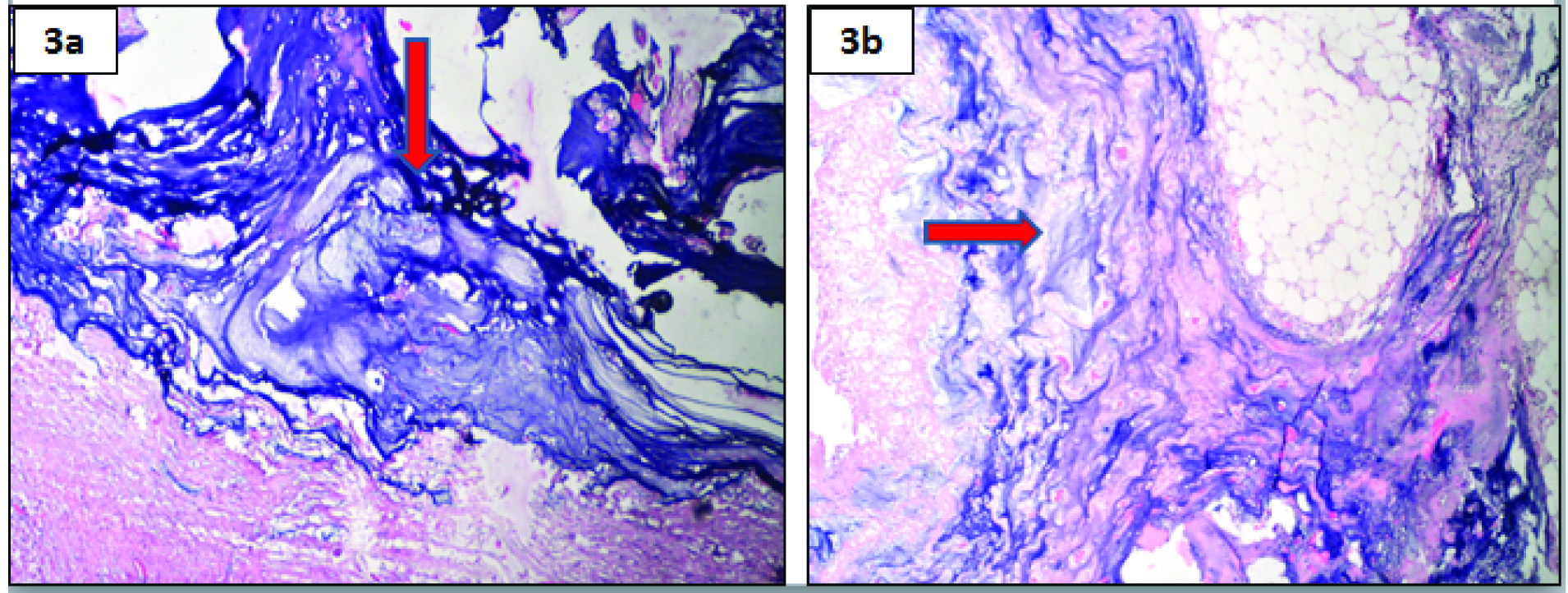
Grossly, the colectomy specimen, gall bladder and spleen were studded with mucin. The omentum was filled with loculated mucinous deposits [Table/Fig-2]. Gall bladder showed multiple black pigmented stones. Microscopically, the peritonectomy specimen showed dissecting pools of mucin with bland looking mucin secreting cells. There was no significant cytologic atypia or epithelial proliferation [Table/Fig-3a]. The serosal aspect of intestine and spleen showed presence of mucin [Table/Fig-3b]. Based on the microscopic features, a diagnosis of Disseminated Peritoneal Adenomucinosis (DPAM) was made.
Grossly omentum was filled with loculated mucinous deposits (arrow).
(a) Microscopically, the peritonectomy specimen showed dissecting pools of mucin (arrow) (H&E, 40x); (b) Serosal aspect of intestine showed presence of mucin (arrow) (H&E, 10X).
The postoperative period was uneventful. The patient was administered 10 cycles of adjuvant chemotherapy with folinic acid, 5-fluorouracil and oxiplatin regimen. The patient is doing well without any recurrence, at the time of publication.
Discussion
PMP, first described by Werth R, is characterized by presence of abundant extracellular mucin in the peritoneum [1]. The incidence in Asia is about one per million per year and is presumed to be about a half of that in the Netherlands and a quarter of that in the United States [2]. Preoperative diagnosis of PMP is difficult due to its varied presentation [3]. Patients usually present with an abdominal mass, increased abdominal girth with abdominal pain [3].
PMP is a poorly understood entity associated with mucin producing tumour [4]. With about 75% of patients are female, most of the cases are of appendiceal origin [5,6]. Other sites like ovary, gallbladder, pancreas, stomach, colon, uterus, fallopian tubes, breast and lung have been reported [6]. The origin of PMP has sometimes been difficult to identify due to rupture of the primary appendiceal tumour or in coexisting appendiceal and ovarian mucinous tumours in females [7]. Depending on the site, the initial presentation of PMP is varied [3]. About 39% of female patients present with ovarian mass, 27% of patients present with suspected acute appendicitis, 23% present with increasing abdominal girth, 25% of male patients and 4% of female patients present with inguinal hernia [3]. New onset hernia is seen in 14% of patients [3]. On physical examination, such patients are indistinguishable from the usual inguinal hernia [8]. Only 4% of patients present with ascites and 9% with non-specific complaints [3]. In the present case, the patient presented initially with right inguinal hernia and then with recurrence as left inguino-scrotal hernia. The appendix was not found intraoperatively, which could be explained by the rupture of the appendiceal mucinous tumour.
Ronnet BM et al., classified PMP into three groups: Disseminated Peritoneal Adenomucinosis (DPAM), Peritoneal Mucinous Carcinomatosis (PMCA) and the intermediate or discordant group (PMCA I/D) [9]. DPAM is composed of epithelium with low grade atypia and minimal architectural complexity in extracellular mucin whereas PMCA is composed of high grade mucinous epithelium with complex architecture with destructive invasion [9]. DPAM has the best prognosis with five year survival rate of 75%. PMCA has five year survival rate of 14% [9].
Pre-operative diagnosis of PMP is difficult. CECT shows the characteristic ‘scalloping effect’ on the surface of the visceral organs due to compression by the viscous mucin and the organising fibrosis [10]. Tumour markers like CEA and CA 19-9 may be frequently elevated. Cytological examination of the ascitic tap may be useful but lack of malignant cells does not rule out malignancy [10].
Management of PMP varies considerably with variable outcome [10]. Debulking surgery, where the peritoneal contents are removed, is the most frequently performed procedure [10]. However, the debulking surgery carries a risk of recurrence [9]. The procedure of choice is the ultraradical surgery proposed by Sugarbaker combined with hyperthermic intraperitoneal chemotherapy [8,11]. It involves multivisceral resection of abdominal organs followed by intraperitoneal chemotherapy with mitomycin C and 5-fluorouracil [8,11]. It is now recommended that just extraction of mucinous tumor and peritoneum from the inguinal canal itself facilitates fibrous closure of the hernia defect [8]. If prior inguinal hernia repair was done, then all tissue and mesh associated with prior herniorrhaphy are resected [8].
Conclusion
PMP is an uncommon entity with varied presentations including new onset inguinal hernia. If not treated properly, patients may present with recurrence of hernia with progressive disease. Hernial contents should be sent for histopathological examination for detection of mucin.
[1]. Werth R, Pseudomyxoma peritoneiArch Gynaecol 1884 24:100-18. [Google Scholar]
[2]. Zhong Y, Deng M, Xu R, Kokudo N, Tang W, Pseudomyxoma peritonei as an intractable disease and its preoperative assessment to help improve prognosis after surgery: A review of the literatureIntractable Rare Dis Res 2012 1(3):115-21. [Google Scholar]
[3]. Esquivel J, Sugarbaker PH, Clinical presentation of the Pseudomyxoma peritonei syndromeBr J Surg 2000 87(10):1414-18. [Google Scholar]
[4]. Ghidirim G, Mishin I, Zastavnitsky G, Pseudomyxoma peritonei presenting with inguinal herniaChirurgia (Bucur) 2011 106(4):527-29. [Google Scholar]
[5]. Hinson FL, Ambrose NS, Pseudomyxoma peritoneiBr J Surg 1998 85(10):1332-29. [Google Scholar]
[6]. O’Connell JT, Tomlinson JS, Roberts AA, McGonigle KF, Barsky SH, Pseudomyxoma peritonei is a disease of MUC2-expressing goblet cellsAm J Pathol 2002 161(2):551-64. [Google Scholar]
[7]. Young RH, Gilks CB, Scully RE, Mucinous tumors of the appendix associated with mucinous tumors of the ovary and pseudomyxoma peritonei. A clinicopathological analysis of 22 cases supporting an origin in the appendixAm J Surg Pathol 1991 15(5):415-29. [Google Scholar]
[8]. Sugarbaker PH, Management of an inguinal hernia in patients with pseudomyxoma peritoneiEur J Surg Oncol 2017 43(6):1083-87. [Google Scholar]
[9]. Ronnett BM, Zahn CM, Kurman RJ, Kass ME, Sugarbaker PH, Shmookler BM, Disseminated peritoneal adenomucinosis and peritoneal mucinous carcinomatosis. A clinicopathologic analysis of 109 cases with emphasis on distinguishing pathologic features, site of origin, prognosis, and relationship to “pseudomyxoma peritonei”Am J Surg Pathol 1995 19(12):1390-408. [Google Scholar]
[10]. Pandey A, Mishra AK, Pseudomyxoma peritonei: disseminated peritoneal adenomucinosis variantBMJ Case Rep 2011 3:2011 [Google Scholar]
[11]. Sugarbaker PH, Cytoreduction including total gastrectomy for pseudomyxoma peritoneiBr J Surg 2002 89:208-12. [Google Scholar]