Opacification of the posterior capsule caused by postoperative proliferation of cells in the capsular bag remains the most frequent complication of cataract surgery with PCIOL implantation [1].
It is a multifactorial physiological consequence of cataract surgery with PCIOL. The development of PCO depends on many factors such as age of patient, preoperative cataract status, diabetes, postoperative inflammation, PCIOL biomaterial and duration of implant in the eye. The choice of surgery and IOL biomaterial are particularly important in relation to eliminating or at least delaying PCO [2].
In recent years, better understanding of the mechanism of PCO formation, advancement in techniques of surgery and introduction of better acrylic IOLs, there has been reduction in incidence of PCO to less than 10%. PCO has the potential to obscure fundus view, thereby, compromising the observation and timely treatment of posterior segment pathologies such as Diabetic Retinopathy (DR) and macular oedema. Attempts have also been made to correlate possible relationship between preoperative cataract status and development of PCO [3].
Currently, there is no known interventional method to get rid of regenerative cells in the equatorial lens bow which makes PCO eradication impossible. It has been reported that acrylic IOLs display the lowest amount of cell proliferation. To best of our knowledge, there is lack of available literature which evaluates the PCO development with the implantation of a single-piece hydrophobic and hydrophilic acrylic IOL in relation to age, preoperative cataract status and diabetes.
Therefore, this study was designed to compare the incidence of PCO formation after cataract surgery with relation to age, preoperative cataract status and diabetes with hydrophobic and hydrophilic IOL implantation after cataract surgery.
Materials and Methods
This prospective observational study was conducted over a period of 12 months from December 2014 to November 2015 at Himalayan Institute of Medical Sciences, Dehradun. After obtaining the formal written approval from ethical committee, the study was conducted as per declaration of Helsinki. Written and informed consent was taken from the patients before including them in the study.
Patients who underwent cataract surgery were included in the study.
Inclusion Criteria
All patients with age related cataract in whom preoperative pupil diameter of 6mm (minimum) could be obtained and who underwent an uneventful cataract surgery were included in the study.
Exclusion Criteria
Patients with complicated cataract, history of prior ocular surgery or inflammation, significant corneal scarring, uveitis or trauma and patients aged less than 40 years were excluded from the study.
Preoperative evaluation was done regarding demographic indices of all patients according to case reporting form; relevant medical history was taken and subjective grading regarding the type of cataract in relation to the lenticular zone of opacification according to The Lens Opacities Classification System III (LOCS III) [4]. Further, the examination included best corrected visual acuity, IOP by applanation tonometry, IOL biometry, slit lamp anterior and posterior segment examination and if posterior segment could not be seen, B-scan ultrasonography was done to rule out any pathology. A single experienced surgeon to remove surgical bias did the operative procedure. The surgical technique included Phacoemulsification followed by “in-the-bag” PCIOL implantation. Surgical steps were the same as that of standard technique [5]. Patients were divided into two groups, in which group I included eyes which underwent cataract surgery with hydrophilic PCIOL and group II included eyes which underwent cataract surgery with hydrophobic PCIOL.
Randomization of patients were done by envelop technique with a person not involved in the study. Post operatively, systemic non steroidal anti-inflammatory drugs were used for pain relief immediately after the surgery. Topical antibiotic along with topical steroid eye drops were given for a period of one month and patients were examined at intervals of one, three and six months to look for development of PCO.
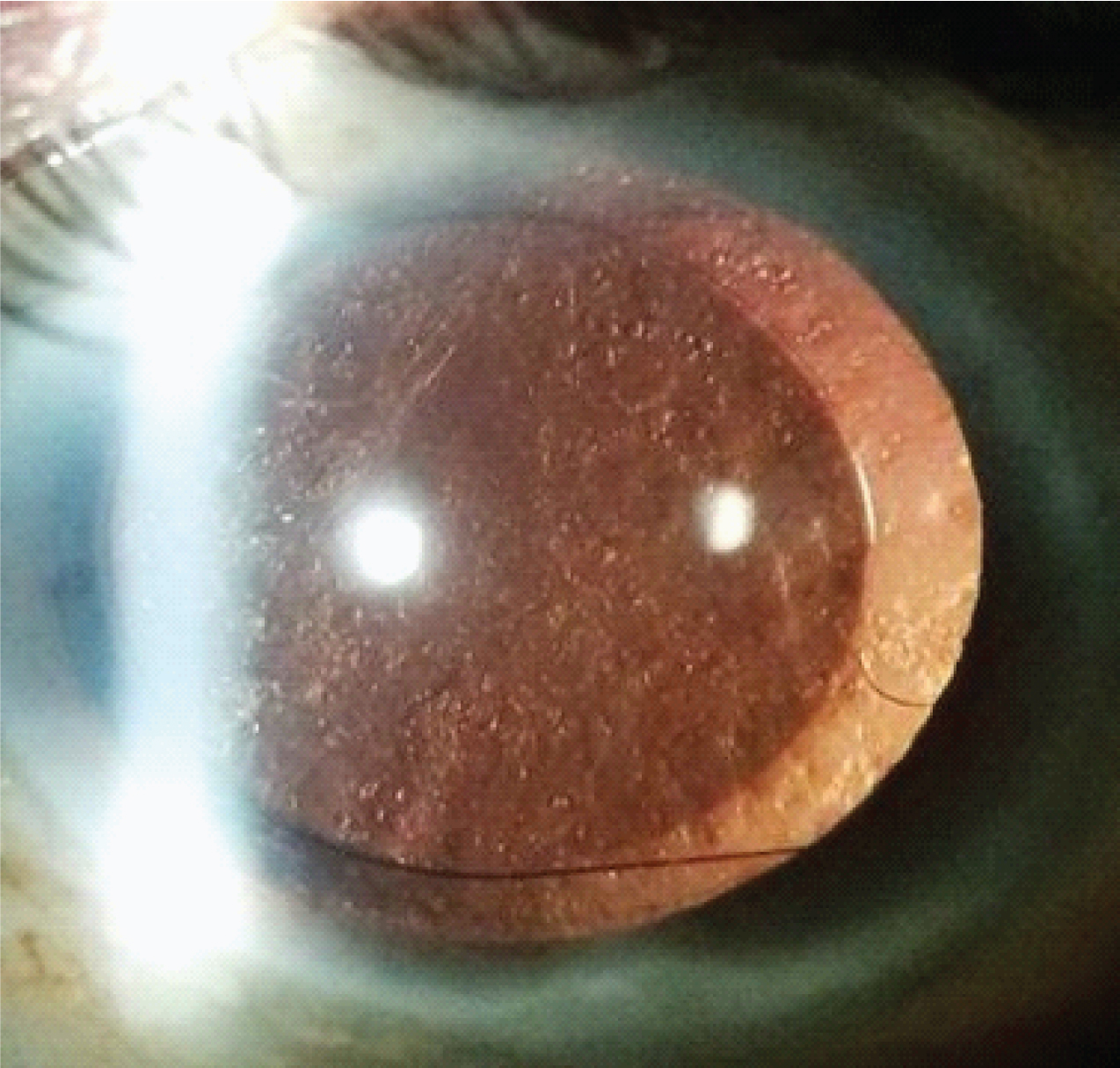
PCO analysis was done on subsequent follow-ups by a single experienced person who was not related to the study and was single blindfolded for patient group to prevent bias. Further analysis was done with the help of retroilluminated images taken with slit lamp guided anterior segment photography (Carl Zeiss Meditec AG, Germany: SL 115 Classic S/N 1093759). PCO was graded in the following manner: Grade 1-No or slight PCO with normal red reflex, no elschnig pearls near the IOL edge. Grade 2-Mild PCO reducing the red reflex, elschnig pearls up to the IOL edge. Grade 3-Moderate fibrosis or elschnig pearls inside IOL edge but with a clear visual axis. Grade 4-Severe elschnig pearls or fibrosis obscuring the visual axis and red reflex [6]. [Table/Fig-1] showing Posterior Capsular Opacification grade 4.
Showing posterior capsular opacification grade 4.
Statistical Analysis
Data were initially entered into an excel spreadsheet and then transferred to SPSS software (Statistical Package for Social Sciences, version 22, SPSS Inc, Chicago, IL). The results were presented in mean±SD and percentages. Chi-square test was used to compare the categorical variables. Binary logistic regression test was used to find the strength of associations. Odds ratio (OR) with its 95% Confidence Interval (CI) was calculated to find the strength of association between dichotomous variables. A p-value<0.05 was considered significant.
Results
A total of 112 eyes of 106 patients were included in the study. Off these, Group I had 55 (49.1%) eyes and group II had 57 (50.9%) eyes. All eyes were further divided according to age. Age 51-70 years consisted 63.6% of eyes in group I and 70.2% of eyes in group II. Age ≤50 years had 12 (21.8%) eyes in group I and 5 (8.8%) eyes in group II, whereas, age >70 years had 8 (14.5%) of eyes in group I and 12 (21.1%) of eyes in group II. The mean age of eyes in group I was 60.22±9.87 years and in group II was 62.56±8.68 years. Data suggests that maximum number of eyes were in the age 51-70 years in both the groups, but there was no statistically significant (p>0.08) difference in age wise distribution of eyes between the 2 groups [Table/Fig-2].
Demographic profile of patients.
| Group I- n (%) | Group II- n (%) | p-value* |
|---|
| No. of patients | 55(49.1%) | 57(50.9%) | |
| Age Groups (years) |
| <50 | 12(21.8%) | 5(8.8%) | 0.08 |
| 51-70 | 35(63.6%) | 40(70.2%) |
| >70 | 8(14.5%) | 12(21.1%) |
| Gender |
| Male | 32(58.2%) | 22(38.6%) | 0.03 |
| Female | 23(41.8%) | 35(61.4%) |
| Diabetes |
| Present | 10(18.2%) | 12(21.1%) | 0.07 |
| Absent | 45(81.8%) | 45(78.9%) |
| Cataract status |
| ≤NO3NC3C3P3 | 45(81.8%) | 50(87.7%) | 0.58 |
| > NO3NC3C3P3 | 10(18.2%) | 7(12.3%) |
*p-value calculated by Chi square test (n=112)
Group I had male dominance with 58.2% and group II showed female dominance with 61.4%. The males were significantly higher in Group I than Group II (p=0.03).
Diabetes was present in 18.2% of eyes in Group I and 21.1% of eyes in Group II. There was no statistically significant (p>0.05) difference in diabetic status between the two groups. Cataract grade ≤ NO3NC3C3P3 (Nuclear Opalescence, Nuclear Color, Cortical, Posterior Subcapsular cataract) was seen in 81.8% eyes in group I and 87.7% eyes in group II while, 18.2% eyes in group I and 12.3% eyes in group II had cataract grade >NO3NC3C3P3. There was no statistically significant (p>0.05) difference in cataract grading between the two groups [Table/Fig-2].
PCO was compared at 6 months postoperatively between two groups. PCO grade 2 was seen to be most common among both Group I (56.4%) and Group 2 (66.7%) eyes. PCO grade 3 was seen among 20 (36.4%) eyes in group I and 10 (17.5%) eyes in group II which was 2.7 times more in group I as compared to group II. Group I had more eyes with severe PCO as compared to group II but the difference was statistically non-significant (p>0.05) [Table/Fig-3].
Association between PCO grades and group I and group II.
| PCOgrade comparison | Group I(n=55) | Group II(n=57) | Odds ratio(OR) (95% CI),*p-value |
|---|
| PCO grades | | | |
| Grade 1 | 4 (7.3%) | 9 (15.8%) | |
| Grade 2 | 31(56.4%) | 38(66.7%) | 0.36 (0.11-1.13), 0.08 |
| Grade 3 | 20(36.4%) | 10(17.5%) | 0.27 (0.07-1.09), 0.06 |
| Grade 4 | 0 | 0 | - |
| According to age | | | |
| Grade 1 | n=4 | n=9 | |
| <50 | 0 | 1(11.1%) | - |
| 51-70 | 2(50%) | 3(33.3%) | 1.66 (0.14-18.87), 0.68 |
| >70 | 2(50%) | 5(55.6%) | |
| Grade 2 | n=31 | n=38 | |
| <50 | 8(25.8%) | 3(7.9%) | 4.00 (0.63-25.02), 0.13 |
| 51-70 | 19(61.3%) | 29(76.3%) | 0.98 (0.24-3.95), 0.98 |
| >70 | 4(12.9%) | 6(15.8%) | |
| Grade 3 | n=20 | n=10 | |
| <50 | 4(20%) | 1(10%) | 2.00 (0.07-51.59), 0.67 |
| 51-70 | 14(70%) | 8(80%) | 0.87 (0.06-11.23), 0.91 |
| >70 | 2(10%) | 1(10%) | |
| According to cataract status | | | |
| Grade 1 | n=4 | n=9 | |
| ≤NO3NC3C3P3 | 0(0%) | 5(55.6%) | NA |
| >NO3NC3C3P3 | 4(100%) | 4(44.4%) | |
| Grade 2 | n=31 | n=38 | |
| ≤NO3NC3C3P3 | 25(80.6%) | 35(92.1%) | 0.35 (0.08-1.56), 0.16 |
| >NO3NC3C3P3 | 6(19.4%) | 3(7.9%) | |
| Grade 3 | n=20 | n=10 | |
| ≤NO3NC3C3P3 | 20(100%) | 10(100%) | NA |
| >NO3NC3C3P3 | 0(0%) | 0(0%) | |
*p-value calculated by binary logistic regression test
PCO - Posterior Capsular Opacification
According to age, comparison of PCO grades between the groups at six months was done. PCO grade 1 was most commonly seen in age>70 years in both groups i.e., Group I (50%) and Group 2 (55.6%), with no significant (p>0.05) difference. There was 2 times more chance of developing PCO grade 3 in group I eyes as compared to group II eyes in age group <50 years. However, there was no significant (p>0.05) difference in PCO grades with age at 6 month between the groups [Table/Fig-3].
Comparison of prevalence of PCO among diabetic patient’s eyes at six months between the groups was done. Among these diabetic patient’s eyes, a higher prevalence of grade 3 PCO was noted in Group I 44 (80%) eyes than Group II 33 (58.3%) eyes. As such, among diabetic patients eyes incidence of PCO grade 3 was noted to be more than PCO grade 2 independent of the two groups.
Comparison of PCO grades with preoperative cataract grade between the groups at six months was also done. Group II had 0.35 times higher chances of developing PCO grade 2 as compared to group I. Among eyes with PCO grade 3; all eyes in both groups had cataract grade ≤ NO3NC3C3P3. There were double the eyes in group I as compared to group II who developed PCO grade 3 [Table/Fig-3].
Discussion
It has been demonstrated in the current study that the incidence of PCO formation is marginally more in eyes implanted with hydrophilic IOLs as compared to eyes implanted with hydrophobic IOLs, and between these two groups, PCO was seen to be more in young individuals, in diabetic population as well as in eyes with preoperative immature cataracts (≤ NO3NC3C3P3) at follow-up period of six months. Although, the higher incidence of PCO formation among hydrophilic IOLs as compared to hydrophobic IOLs has been well investigated in depth to date, it must now be interpreted in light of patient factors such as age, diabetes and preoperative cataract status.
In present study, the hydrophilic IOL was associated with a higher incidence of PCO formation than the hydrophobic IOL at six months after cataract surgery. Both IOLs had a single piece design, square-edged optics and no haptic angulation. Kugelberg M et al., in 2006 reported that patients with the hydrophilic acrylic IOL had a significantly greater percentage area and severity of PCO than those with the hydrophobic acrylic IOL one year after surgery, which is consistent with present study. A material’s hydrophilicity is inversely proportional to the contact angle. The hydrophobic IOL therefore has a higher contact-angle measurement than the hydrophilic IOL, which might make it more difficult for Lens Epithelial Cells (LECs) to migrate on the hydrophobic IOL surface. The protective influence of the square-edged design with respect to PCO seems to be more pronounced when the material is hydrophobic [7]. Vasavada A et al., also, reported that PCO was significantly less with the hydrophobic acrylic IOL at three years period after cataract surgery [8].
In present study, it was seen that chances of developing grade 3 PCO was more among patients with presenile cataract i.e., in patients younger than 50 years. This could be explained on the basis of the fact that in patients of younger age group, the LECs have more proliferative capacity as well as the inflammatory response is higher in them, therefore, causing increased tendency for PCO formation. This was in accordance to a study done by Jamal Solomon L et al., who concluded that younger age was a significant risk factor for developing PCO [9]. Similarly, in a review done by Pandey SK et al., younger age was mentioned as a potential risk factor for the development of PCO [10]. In present study, we also compared the incidence of developing PCO between hydrophilic and hydrophobic IOLs according to age and we found that there was an increased risk by 2 times of developing grade 3 PCO among eyes with hydrophilic IOLs in age group of less than 50 years. This could be explained on the basis of more proliferative nature of LECs in young individuals and less adhesive nature of hydrophilic IOLs to stop PCO formation. No similar study to the best of our knowledge has been published till date comparing incidence of PCO formation between IOL biomaterial with age being the confounding factor.
In present study, it was seen that chances of developing grade 3 PCO was more among diabetic patients at six months follow-up. It is well known that blood aqueous barrier of eyes of diabetic patients is compromised and any surgical invasion further damages it. This causes increased post operative inflammation and thus, increased proliferation of LECs leading to formation of PCO. Hayashi K et al., reported significantly greater chances of developing PCO in diabetics than in controls at 3 years after surgery [11]. In another study done by Praveen M et al., they concluded that the duration of diabetes increased the incidence of developing PCO at 4 years [12]. In present study, we also noted that risk of developing PCO was marginally higher in eyes implanted with hydrophilic IOLs as compared to eyes implanted with hydrophobic IOLs. The results of this study also confirmed that cataract grade >NO3NC3C3P3 (mature cataract) had a lower tendency to produce PCO. The fact that mature cataracts carry lower incidence of PCO formation could be explained by an alteration in the anterior lens epithelium, which is normally responsible for regenerative capabilities of lens equatorial cells. A few reports have suggested that senile complete cataracts (mature cataracts) had a significantly lower tendency to produce postoperative capsular opacification than other cataract types (nuclear, cortical, posterior subcapsular) [13], which is in accordance with present study. In present study we also compared the incidence of developing PCO between hydrophilic and hydrophobic IOLs according to preoperative cataract grade and we found that there was 2 times increased risk of developing grade 3 PCO among eyes with hydrophilic IOLs. To the best of our knowledge this is the first study comparing incidence of PCO formation between IOL biomaterial with preoperative cataract grade being the confounding factor.
Limitation
Study was conducted for a short follow-up period since, development of PCO is minimal at six months period following cataract surgery with PCIOL implantation. A longer follow up would have given more accurate results.
Conclusion
The incidence of PCO was higher with the hydrophilic IOL. However, the increased incidence of PCO can also be attributed to age of patient, diabetes mellitus and cataract status, factors known to be associated with higher incidence of PCO.
*p-value calculated by Chi square test (n=112)
*p-value calculated by binary logistic regression test
PCO - Posterior Capsular Opacification