As advancements are made in radiological modalities and surgical treatments for pancreatic cancer, more and more cystic lesions of the pancreas are identified and resected. Pancreatic cystic neoplasms represent approximately 15% of all pancreatic tumours [1]. SCN is a type of neoplasm with very low malignant potential [2] and accounts for 30% of all pancreatic cystic neoplasms [3] and are more frequently found in female patients (approximately 75%) with a mean age of 60-80 years [2]. SCN is currently deemed as a benign lesion, and observation instead of surgical resection is considered as first line treatment for asymptomatic patients with stationary tumour size [4].
Patients who are diagnosed with either SCN or MCN typically do not show any symptoms. If symptoms exist, they are often non specific, such as abdominal pain or some other vague discomfort of the gastrointestinal system [2].
The preoperative distinction between SCN and MCN is crucial due to their completely different treatment strategies. Currently, several diagnostic tools are used such as Computed Tomography (CT) and Magnetic Resonance Imaging (MRI). Zhang W et al., also demonstrated that combination of Endoscopic Ultrasound with Fine Needle Aspiration (EUS-FNA) could increase the preoperative diagnostic rate for SCN and MCN [8]. However, these current diagnostic tools are still unsatisfactory for the differentiation of SCN and MCN [9]. In this study, we aimed to evaluate whether clinical parameters could provide some benefits to diagnose pancreatic cystic neoplasms more correctly before operation, thus to prevent unnecessary operation.
Materials and Methods
This retrospective study was approved by the Institutional Review Board (IRB number:103-5008C).
From 1992 to 2010 at Chang Gung Memorial Hospital, Linkou, and from 1988 to 2014 at Chang Gung Memorial Hospital, Keelung, 141 patients with pathologically confirmed SCN or MCN underwent pancreatic resection. Patients with mucinous cystoadenocarcinoma were excluded. When the pathology showed that MCN or SCN occurred together with other cystic tumours, such as Intraductal Papillary Mucinous Neoplasm (IPMN), these patients were also excluded. We only enrolled patients with complete data records.
A total of 36 patients with SCN and 47 patients with benign MCN (cystadenoma and non invasive proliferative MCN) were included in this study. We retrospectively reviewed the demographic and clinicopathologic factors of the patients. With respect to the pathological findings, the SCN was distinguished by cuboidal epithelial cells with clear cytoplasm that line its cystic wall, while MCN was distinguished by columnar mucinous cells that line its cystic wall [10].
Statistical Analysis
Mann-Whitney U test was used to compare these two groups in terms of numerical factors, including age, total bilirubin, direct bilirubin, albumin, AST, ALT, ALP, amylase, lipase, Carcinoembryonic Antigen (CEA), Cancer Antigen (CA) 19-9, overall survival, and size. A Chi-square test was applied for comparisons between the nominal factors from the two groups, including gender, symptoms and locations. Then, stepwise logistic regression was used to explore the independent factors. Additionally, a ROC curve was used and the AUC was calculated. For any determination of the cut-off value, Youden’s index was calculated and applied. Positive Predictive Value (PPV), Negative Predictive Value (NPV), and the accuracy of the tests in the prediction of SCN or MCN lesions were calculated. These statistical analyses were conducted with the Statistical Package for Social Sciences (SPSS) software (version 17.0; SPSS, Chicago, IL, USA). All tests were two-sided, and p<0.05 was considered statistically significant.
Results
The median age of the patients in our study cohort was 61.5 years in the SCN group and 45 years in the MCN group. In all, 86.1% of the patients in the SCN group and 87.2% of the patients in the MCN group were female. Operative procedures included Whipple’s operation, distal pancreatectomy, subtotal pancreatectomy, and enucleation, which were based on tumour location. [Table/Fig-1] shows the demographic data of both the SCN and MCN groups. Patients with MCN were generally younger than patients with SCN (p=0.001). In our study cohort, no difference was observed in the gender distribution because both groups were predominantly female. The AST level was higher in the SCN group (p=0.009), the ALT level was higher in the SCN group (p=0.032), the direct bilirubin level was higher in the SCN group (p=0.0138), and the ALP was higher in the SCN group (p<0.001). On the contrary, the level of albumin was higher in the MCN group (p=0.043). In regard to tumour size, patients in the MCN group were noted to have relatively larger tumours (mean size of 8.11±4.72 cm vs. 6.05±3.58 cm, p=0.022). The SCNs were located predominantly in the head of the pancreas whereas the MCNs were predominantly located in the tail and body (p<0.001).
Univariate analysis and logistic regression.
| Factors | Univariate analysis | Logistic regression |
|---|
| SCN(n=36) | MCN(n=47) | p | p* |
|---|
| Age (years) | 59.61±15.39‡ | 48.36±14.48 | 0.001 | n.s. |
| Gender (M/F) | 5 / 31 | 6 / 41 | 0.566 | n.s. |
| Bilirubin Direct | 0.57±0.77 | 0.23±0.21 | 0.0138 |
| Bilirubin Total | 0.97±0.87 | 0.69±0.35 | 0.074 |
| AST (U/L) | 30.25±23.28 | 19.16±10.37 | 0.009 | n.s. |
| ALT (U/L) | 33.13±44.36 | 17.18±12.97 | 0.032 | n.s. |
| Albumin (U/L) | 4.01±0.75 | 4.37±0.55 | 0.043 | n.s. |
| ALP (U/L) | 87.79±61.16 | 54±17.04 | <0.001 | 0.044 |
| Amylase (U/L) | 93.90±69.37 | 129.2±98.61 | 0.205 | n.s |
| Lipase (U/L) | 146.78±236.33 | 197.04±406.81 | 0.95 |
| CEA (ng/ml) | 2.33±1.35 | 2.25±2.95 | 0.059 |
| CA 19-9 (U/ml) | 39.11±69.73 | 22.74±24.32 | 0.279 |
| Overall survival (months) | 46.83±47.26 | 50.39±46.26 | 0.61 |
| Symptoms (Y/N) | 21/9 | 36/11 | 0.35 |
| Size (cm) | 6.05±3.58 | 8.11±4.72 | 0.022 | n.s. |
| Location in pancreas (%) | |
| Head | 17 (48.6) | 2 (4.3) | <0.001 | 0.013† |
| Body | 12 (34.3) | 14 (30.4) |
| Tail | 6 (17.1) | 30 (65.2) |
* Only factors with statistical significance in univariate analysis were enrolled; †Head location was compared with body and tail location; ‡for each continuous variable, standard deviation was added; SCN=Serous Cystic Neoplasm; MCN=Mucinous Cystic Neoplasm; AST= Aspartate Transaminase; ALT=Alanine Transaminase; ALP=Alkaline Phosphatase.
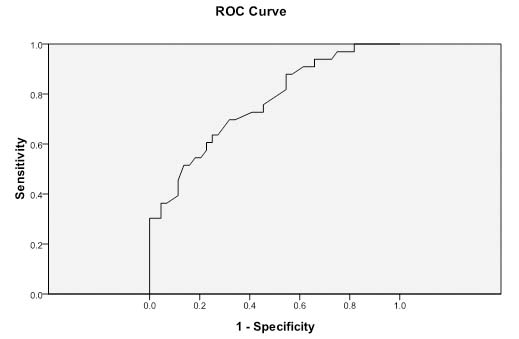
After a multivariate analysis by stepwise logistic regression, it was determined that the independent factors were the ALP level and the tumour location. We then used an ROC curve [Table/Fig-2] with an AUC of 0.762 to define the cut-off value of the ALP level for the distinction of SCN from MCN. The cut-off value for ALP was 61.5 U/L with a sensitivity of 0.636 and a specificity of 0.75.
ROC curve for alkaline phosphatase.
ROC curve and cut-off value for alkaline phosphatase with AUC=0.762. The optimal cut-off value= 61.5. Alkaline phosphatase (ALP); ROC=receiver operating characteristic; AUC=area under the curve.
When the ALP level and the tumour location (proximal pancreas) were combined for SCN prediction, the predictive power of our explored factors were determined to have a sensitivity of 66.7%, a specificity of 84.2%, a PPV of 40%, and an NPV of 94.1. The overall accuracy of prediction was 81.8% for the differentiation of SCN and MCN according to our study results [Table/Fig-3]. As to the location in the non proximal pancreas with ALP levels >61.5 U/L, the predictive power had a sensitivity of 75.6%, a specificity of 50.0%, a PPV of 79.5%, an NPV of 44.4%, and an accuracy of 68.4% for the differentiation of SCN and MCN.
Predictive power under the condition of ALP >61.5 U/L.
| DiagnosisLocation | Sensitivity(%) | Specificity(%) | PPV(%) | NPV(%) | Accuracy(%) | LR+(%) | LR-(%) |
|---|
| Proximal pancreas | 66.7 | 84.2 | 40 | 94.1 | 81.8 | 4.22 | 0.39 |
| Body and tail of pancreas | 75.6 | 50 | 79.5 | 44.4 | 68.4 | 1.51 | 0.49 |
PPV: Positive Predictive Value; NPV: Negative Predictive Value; LR+: Likelihood Ratio for a positive test; LR-: Likelihood Ratio for a negative test.
Discussion
In this study, we found that both serum ALP level and tumour locations could be used as ancillary factors to help predict the nature of pancreatic cystic neoplasms. When patients diagnosed with pancreatic cystic neoplasms have ALP level over 61.5 U/L and pancreatic cystic neoplasm proximally located, SCN is indicated (84.2% specificity and 94.1% negative predictive value), but otherwise, MCN may be implicated.
It has been reported that the anatomical distributions of SCN and MCN are different with SCN predominantly located in the proximal pancreas [5] and MCN primarily located in the body or tail of the pancreas [6,7,11]. In our study, SCNs were found to be located mainly in the head of the pancreas (SCN: 48.6% vs. MCN: 4.3%) and MCNs were predominantly located in the tail and body (SCN: 51.4% vs. MCN: 95.6%) (p<0.001), in line with the previous studies [6,7,11]. According to the previous study, MCNs are usually diagnosed in middle-aged (40-50 years) female patients [6], whereas SCNs are often diagnosed in old-aged (60-80 years) female patients [2,3]. In our study, we also found this trend. Our result shows that the mean age of the patients with MCNs or SCNs was 48.36±14.48 or 59.61±15.39 years, respectively [Table/Fig-1]. Both MCNs and SCNs were female predominant (female to male ratio: MCN: 87.2%, SCN: 86.1%) [Table/Fig-1]. However, both age and sex could not be independent factors to distinguish MCNs from SCNs after multivariate analysis in our study.
CA 19-9 is widely used as a pancreatic cancer tumour marker. Sperti C et al., found that serum CA 19-9 level was elevated in MCNs (mean: 450.6 U/mL) but within normal range in SCNs [12]. In our study, however, we found that both CEA and CA 19-9 could not be predicting factors of pancreatic cystic neoplasms.
ALP contains a group of hydrolase enzymes which are responsible for hydrolysis of phosphate esters to generate an organic radical and inorganic phosphate. ALP can be found in the bone, liver, intestines, and in the duct system, islet cells, and acini of the pancreas [13,14]. It has been shown that ALP could be used as poor prognostic factor to predict the prognosis of patients with pancreatic ductal adenocarcinoma after surgery [15,16]. About MCNs and SCNs, the role of ALP has not been investigated yet. In our study, the ALP level is higher in SCN groups than MCN group (p<0.001) [Table/Fig-1]. After we applied the ROC curve [Table/Fig-2] to analyse, the cut-off ALP value of 61.5 U/L, with an AUC of 0.762, was determined.
According to our results, as we took tumour location and ALP level into consideration, a cystic lesion in the proximal pancreas with an ALP level 61.5 U/L may be an SCN with NPV of 94%. This high NPV may indicate our findings as a screening test. Once diagnosis of SCN is excluded, surgical treatment may be considered.
Limitation
This study, however, does have some pitfalls. First, this is a retrospective study and only patients with a definite pathological diagnosis (based on surgical specimens) were enrolled. For patients who were classified as low risk according to current clinical guidelines (e.g., Fukuoka guideline) [17], the pathological diagnosis was unknown, and therefore, we did not enroll them in this study. Second, only a small population was available for this retrospective study. Additional prospective validation should be conducted in order to further verify its clinical impact. Third, the possible extrahepatobiliary sources of ALP were not taken into consideration, such as bone, although we had eliminated possible confounding effect by logistic regression. Moreover, heterogeneity exists in such a small study population although we adopted Mann-Whitney U-test instead of t-test. Finally, we did not evaluate the predictive power of the combination of the ALP level with other specific imaging findings or with the results of a cytological exam (such as the size of the pancreatic duct and the components of the cystic content, etc.).
Although we utilized multivariate analysis (logistic regression) to exclude the confounding effect caused by bilirubin level, AST, and ALT, it would be better to find a solid and scientific evidence to prove the elevation of ALP mainly related to these tumors. For this concern, some laboratory techniques, such as stain of immunohistochemistry for ALP, may provide another point of view to independently evaluate the result of this retrospective study in addition to statistical strategies.
Conclusion
SCN and benign MCN may be not easily distinguished preoperatively even with the aid of current imaging modalities. Based on our analysis, we suggested a simple clinical laboratory test, the level of ALP, combined with the tumour location, may be equipped with some potential for differential diagnosis. Further studies with stronger power of statistical analysis and scientific ground are needed in order to validate our current result.
* Only factors with statistical significance in univariate analysis were enrolled; †Head location was compared with body and tail location; ‡for each continuous variable, standard deviation was added; SCN=Serous Cystic Neoplasm; MCN=Mucinous Cystic Neoplasm; AST= Aspartate Transaminase; ALT=Alanine Transaminase; ALP=Alkaline Phosphatase.
PPV: Positive Predictive Value; NPV: Negative Predictive Value; LR+: Likelihood Ratio for a positive test; LR-: Likelihood Ratio for a negative test.