Glucose-6-Phosphate Dehydrogenase (G6PD) enzyme deficiency is the most common inherited genetic disorder affecting RBCs in humans. The disorder is characterised by inability of RBC to maintain a balanced redox state when challenged by oxidative stresses like drugs, infections and certain food substances leading to severe haemolytic anaemia which complicates any therapeutic management in these patients. This article reports on a successful endodontic management of a 36-year-old class III G6PD deficient male patient with deep carious lesion in left mandibular first molar tooth (36). Considering there is no protocol precedence in dental literature, in consultation with the physician/haemotologist a three step protocol for safe and efficient dental management is proposed.
Acute haemolytic anaemia, Endodontic therapy, Favism, G6PD deficiency, Red blood cells
Case Report
A 36-year-old male patient reported to a private dental office with acute pain in left mandibular region. Based on the symptoms, clinical findings (deep cavitated lesion), diagnostic tests (percussion test, pulp sensitivity test) and investigations (IOPA) [Table/Fig-1] it was diagnosed as a case of symptomatic irreversible pulpitis with apical periodontitis in tooth 36 and the treatment plan was endodontic therapy in relation to 36 followed by post-endodontic restoration.
His earlier medical report stated that he was suffering from class III G6PD deficiency and was advised to undergo any invasive procedure only after consulting with his haematologist. It further stated that, he was advised to avoid a list of drugs (mostly analgesics and antibiotics) and certain food substance (fava beans, windsor beans, broad beans). Dental treatment as such was not contraindicated for him. After a detailed discussion with the patients’ haematologist, it was decided to perform a two-visit endodontic procedure in tooth 36 under local anaesthesia with patient’s consent.
In the first visit pulpal anaesthesia in tooth 36 was gained by conventional Inferior Alveolar Nerve Block (IANB) using 2.5 mL of 2% lignocaine with adrenalin (1: 2, 00,000) (LIGNOX 2% A, Indoco Remedies Ltd., Gujarat, India). Instrumentation was performed with manual K files (Dentsply Maillefer) by crown down pressureless technique. The canals were irrigated sequentially with every change of file using 5 mL of 3% sodium hypochlorite (Parcan, Septodont Healthcare India Pvt., Ltd., Raigad, Maharashtra, India), 5 mL of 17% EDTA (Pulpdent corporation, Watertown, Massachusetts, USA) for 60 seconds followed by 5 mL of saline (Baxter India Pvt., Ltd., Mumbai, India) for 120 seconds. Cavity was temporarily restored with type II GIC (G.C. corporation, Tokyo, Japan) restoration after using Ca(OH)2 (Sultan healthcare Inc, Englewood, NJ, USA) as intracanal medicament. The patient was not prescribed any drugs.
During the second visit (15 days later) the access cavity was reopened, canals were irrigated with 5 mL of 2% Chlorhex (CHX) (ASEP_RC, Stedman Pharma Pvt., Ltd., Chennai, India) for 60 seconds followed by 10 mL of saline for 120 seconds (neutralise the effect of CHX) as final irrigant. No instrumentation was carried out at this sitting. In the absence of clinical signs and symptoms it was decided to obturate the canals. Obturation was done using 2% Gutta Percha (GP) points (Dentsply Maillefer) and ZnOE (Dental products of India, Mumbai, India) as sealer by cold lateral condensation method. Size of the master cone was 40 for distal canal, 30 for mesiobuccal and mesiolingual canals [Table/Fig-2]. Coronal seal was provided using high strength posterior GIC (G.C. corporation, Tokyo, Japan) as amalgam/resin were avoided as precautionary method. The tooth received a full coverage crown one month after obturation and was reviewed after six months [Table/Fig-3], one year [Table/Fig-4]. The tooth exhibited satisfactory healing.
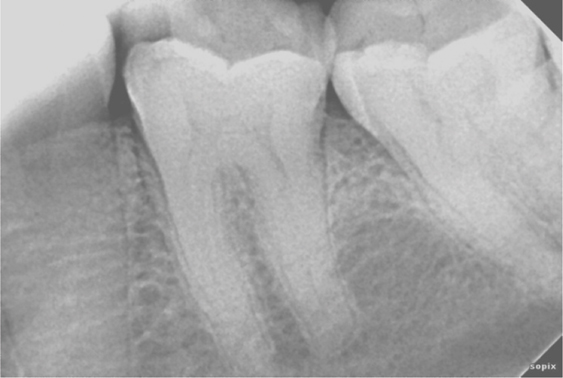
Preoperative peri-apical radiograph of tooth 36 showing widening of periodontal ligament space and peri-apical radiolucency.
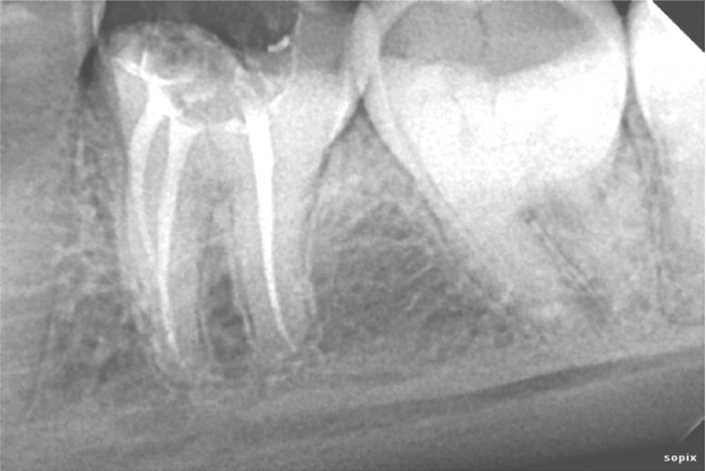
Peri-apical radiograph of tooth 36 immediately after obturation.
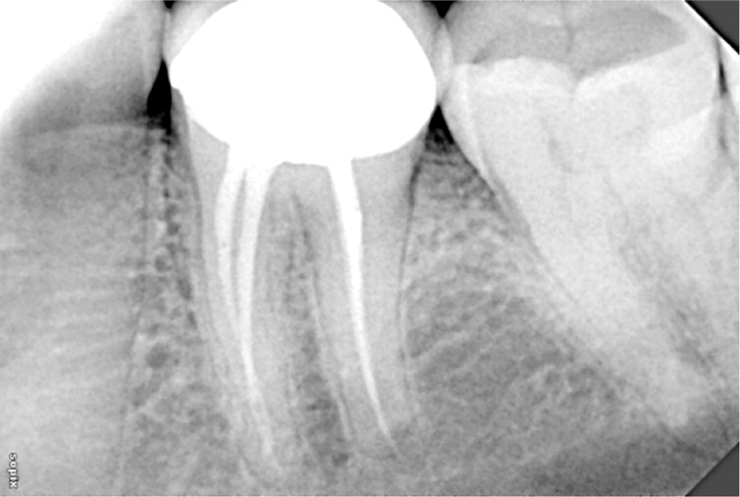
Peri-apical radiograph of tooth 36, six months after obturation.
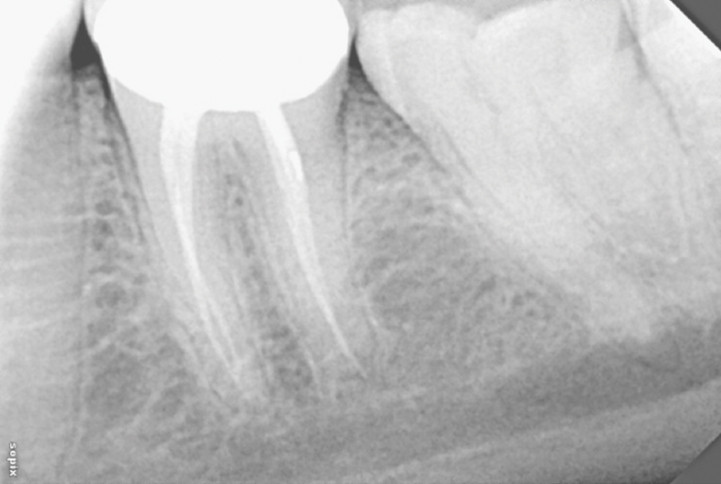
Peri-apical radiograph of tooth 36, one year after obturation.
Discussion
G6PD deficiency is the most common enzymatic disorder affecting the red blood spectrum of cells in humans [1]. Approximately 400 million people are affected by this deficiency [2,3]. Although reported to have a geographical distribution similar to that of falciparum malaria, now its prevalence is worldwide [2,3]. The deficiency is a cystosolic X-linked hereditary genetic defect caused by mutations in the G6PD gene. Typically the inheritance is X-linked with men being affected hemizygously [4].
WHO has classified G6PD deficiency into five different classes based on the level of enzyme deficiency [1]. Class IV and V are not significant with residual enzyme activity of 100% and >100% respectively, while class I (<10%) represents the most severe congenital non spherocytic form of deficiency, Class II and class III exhibit <10%, 10 - 60% residual enzyme activity respectively [1].
While there is concurrence among researchers that ingestion of fava beans and infections by a variety of organisms can lead to acute haemolysis in G6PD deficient patients [3], there is considerable debate among researchers on which drugs should be avoided in G6PD deficient individuals, especially local anaesthetics, NSAID’s and antibiotics commonly used by dentists.
Drug induced acute haemolysis in G6PD deficient individuals formed the basis for its discovery (primaquine senstivity trials) [1] and is considered the most common clinical consequence of G6PD deficiency [5]. Many compounds/drugs have been added and subsequently removed over the years from the list of prohibited medications. In fact G6PD deficiency became a prototype study case in pharmacogenetics [6]. Local anaesthetic agents prilocaine, articaine, lidocaine and surface anaesthetic benzocaine have been reported to induce methaemoglobinemia in G6PD deficient individuals [2,3].
Performing dental therapy on such patients becomes challenging because of the speculation that the procedure by itself may lead to oxidative stress triggering Acute Haemolytic Anaemia (AHA). A thorough search of the Medline/Pubmed, Cochrane library and Scopus literature database revealed only three direct case reports of dental management in G6PD deficiency individuals [2,4,7] which too did not propose any safe protocol for any general dental management under local anaesthesia. Considering these factors, a three-step protocol is proposed for any dental therapy under L.A in G6PD deficient individuals irrespective of the class (risk category).
First step in the protocol is to thoroughly screen the patients. This includes a comprehensive case history and a detailed blood investigation. A clue to the underlying disorder usually comes from family history or from a previous history of haemolysis to any of the trigger factors. A complete blood picture analysis is a must to rule out any additional abnormality. In suspicious cases G6PD screening test (fluorescent spot test) should be carried out followed by spectrophotometric assay to ascertain the level of deficiency [8]. Haematologist consultation is must, especially in case of any modification in typical treatment strategy. First step in the protocol will help immensely to assess the level of deficiency and to know about the patient’s trigger factors.
The second step in the protocol is to prevent oxidative stress as a result of therapeutics. In the present case, the haematologist cautioned about the use of analgesics, antibiotics, local anaesthetics, sodium hypochlorite, EDTA, CHX, Ca(OH), ZnOE and GP. No antibiotics/analgesics were prescribed. Since there was no alternative to the use of local anaesthesia and to irrigants, obturating/restorative materials, they were used in low dosage (anaesthetics and irrigants) because the severity of AHA is dose dependent [9].
A two-visit endodontics will be a better option than single visit endodontics (to reduce the chances of flare-up). A strict adherence not to violate the apical constriction, extrude debris, irrigant beyond periapex is a must procedurally, since it will prevent inoculating microbiota and their toxins in periapex. Crown down pressureless technique will be the preferred instrumentation technique as it will satisfy the above said criteria [10].
The third step in the protocol is to continuously monitor the patient for five days followed by periodic monitoring for 30 days after the visits as suggested by the haematologist. Clinicians should be very much aware about the clinical signs and symptoms of an acute haemolytic crisis. The crisis typically starts 24 to 72 hours after exposure and can extend up to seven days if not diagnosed early [3]. Usually AHA is self limiting and regresses once the offending agent is withdrawn. In severe cases hospitalisation followed by blood transfusion may be necessary. Cyanosis, headache, dyspnoea, fatigue, lumbar/substernal pain, jaundice, dark urine is other associated clinical signs and symptoms. Pathological findings include normocytic and normochromic anaemia, reticulocytosis, transient presence of Heinz bodies on peripheral smear leading to bite cells, elevated levels of bilirubin, urobilinogen, lactase dehydrogenase and very low levels of heptoglobin [3,8]. It is better to inform patients in advance about the signs/symptoms especially in high risk patients since endodontic and other dental therapy is performed in individuals as an outpatient procedure. A complete blood picture analysis is mandatory, three days after the dental procedure to rule out any haematological changes.
Conclusion
Drug induced haemolysis in G6PD deficiency definitely presents many clinical challenges in dental therapeutics. The awareness of the patient to this condition helped in managing his dental problem and in formulating evidence based clinical protocol which will immensely benefit clinicians in safe and efficient dental management in G6PD deficient patients.
[1]. Beutler E, G6PD DeficiencyBlood 1994 84:3613-36. [Google Scholar]
[2]. Hernández-Pérez D, Butrón-Téllez Girón C, Ruiz-Rodríguez S, Garrocho-Rangel A, Pozos-Guillén A, Dental considerations in children with glucose-6-phosphate dehydrogenase deficiency (favism): A review of the literature and case reportCase Reports in Dentistry 2015 2015:506459 [Google Scholar]
[3]. Elyassi AR, Rowshan HH, Perioperative management of the glucose-6-phosphate dehydrogenase deficient patient: A review of literatureAnaesthesia Progress 2009 56:86-91. [Google Scholar]
[4]. Tosun G, Sener Y, Apert syndrome with glucose-6-phosphate dehydrogenase deficiency: A case reportInternational Journal of Paediatric Dentistry 2006 16:218-21. [Google Scholar]
[5]. Youngster I, Arcavi L, Schechmaster R, Akayzen Y, Popliski H, Shimonov J, Medications and glucose-6-phosphate dehydrogenase deficiency: An evidence-based reviewDrug and safety 2010 33(9):713-26. [Google Scholar]
[6]. Luzzatto L, Seneca E, G6PD deficiency: A classic example of pharmacogenetics with on-going clinical implicationsBritish Journal of Haematology 2014 164:469-80. [Google Scholar]
[7]. Quereshy FA, Gold ES, Powers MP, Hemolytic anaemia in a glucose-6-phosphatede hydrogenase-deficient patient triggered by a maxillofacial infectionJournal of Oral Maxillofacial Surgery 2000 58:805-07. [Google Scholar]
[8]. Luzzatto L, Poggi V, Glucose-6-Phoshate Dehydrogenase Deficiencyn: Nathan and Oski’s Haematology and oncology of infancy and childhood 2015 8th edPhiladelphia, USA:609-629. [Google Scholar]
[9]. Luzzatto L, Nannelli C, Notaro R, Glucose 6 phosphate dehydrogenase deficiencyHaematology Oncology Clinical N Am 2016 30:373-93. [Google Scholar]
[10]. Morgan LF, Montgomery S, An evaluation of the crown down pressureless techniqueJournal of Endodontics 1984 10:491-98. [Google Scholar]