MCs are specialized cells of haematopoietic origin distributed ubiquitously in the connective tissue. They are common residents of human body both in physiologic and pathologic conditions [1]. The paramount features of these cells include the presence of granularity in their cytoplasm. Upon degranulation, they release chemical mediators like histamine, heparin, certain chemokines and enzymes which can mediate inflammatory reactions [2]. The role of MCs in various pathologies have been elucidated over time which has enlightened us with new knowledge and understanding of these specialized cells [3]. In oral cavity, MCs are seen in all soft tissues including dental pulp [4]. Most of the pathologies occurring in the oral cavity may preclude chronic inflammation and therefore are a habitat for MCs [4].
Identification of these cells is best done with metachromatic stains. Metachromasia is the property by which the tissue dye complex exhibits a shift in absorption of light towards shorter wavelength with an inverse shift in colour transmission or emission towards the longer wavelengths. As a result the colour of the tissue bound dye complex differs significantly from the colour of the original dye complex [5]. The compounds responsible for metachromasia are called chromatophores and in case of MCs, it was identified as heparin [6]. Routinely used metachromatic stain in a laboratory setting is toluidine blue (tolonium chloride). Other metachromatic stains include methyl violet, azure A, azure B, safranin etc., [6]. Metachromatic dyes stain MC granules a deep purple colour, with the background and nucleus staining blue colour [5].
The aim of this comparative pilot study was to evaluate and compare the number of intact and degranulated MCs in IFH, OPG, OLP and OSCC with NOM using azure A and toluidine blue. This study also focuses to determine the efficiency of azure A as a metachromatic stain in identifying MCs in comparison to toluidine blue as a control.
Materials and Methods
This comparative pilot study was conducted in the Department of Oral Pathology and Microbiology, Annoor Dental College and Hospital, Kerala, India during the period of November 2013 to January 2014. Previously histopathologically proven, five cases each of OPG, OLP, IFH and OSCC were retrieved from the departmental archive. All these tissue sections were formalin fixed and paraffin embedded. Normal oral mucosal tissues biopsied from extraction sites were used as controls. Two sections, each of 3 μ thickness were cut; one stained with 1% toluidine blue, the other stained with azure A for demonstrating MCs. Modified Kramer and Windrum technique was used for the preparation of azure A solution [5]. Fifteen fields (40X) from each of these slides were selected randomly and photographed using microscope camera Canon Canoscan LiDE. These images were later transmitted to a computer monitor and the cells were counted using Image J 1.43 software to avoid human error. The MC count, both intact and degranulated cells were computed separately and expressed as the number of MCs per high power field.
Criteria for Identification and Counting of Mast Cells
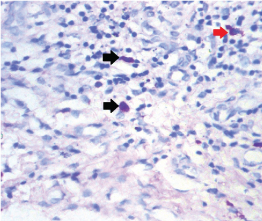
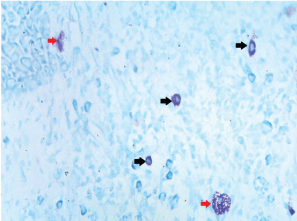
The cytoplasmic granules in MCs are difficult to observe in Haematoxylin and Eosin (H and E) stained sections and can only be definitely recognized with metachromatic stains. With these stains, the intact cells appear as spindle to ovoid shaped cells with purplish red granules in the cytoplasm and a unilobar spherical sky blue coloured nuclei placed at the center or eccentrically. Those MCs, which showed no disruption of their surface membranes, were estimated as intact cells and those which showed partial or complete discontinuity of their cell membranes with one or more extruded purple staining granule(s) were counted as degranulated MCs [Table/Fig-1,2] [7]. As it is a comparative pilot study, statistical analysis was not done and only the mean total and standard deviation was calculated and compared.
Intact and degranulated mast cells seen in sections stained with toluidine blue 40X (black: intact cell; red: degranulated cell).
Intact and degranulated mast cells seen in sections stained with azure A 40X (black: intact cell; red: degranulated cell).
Results
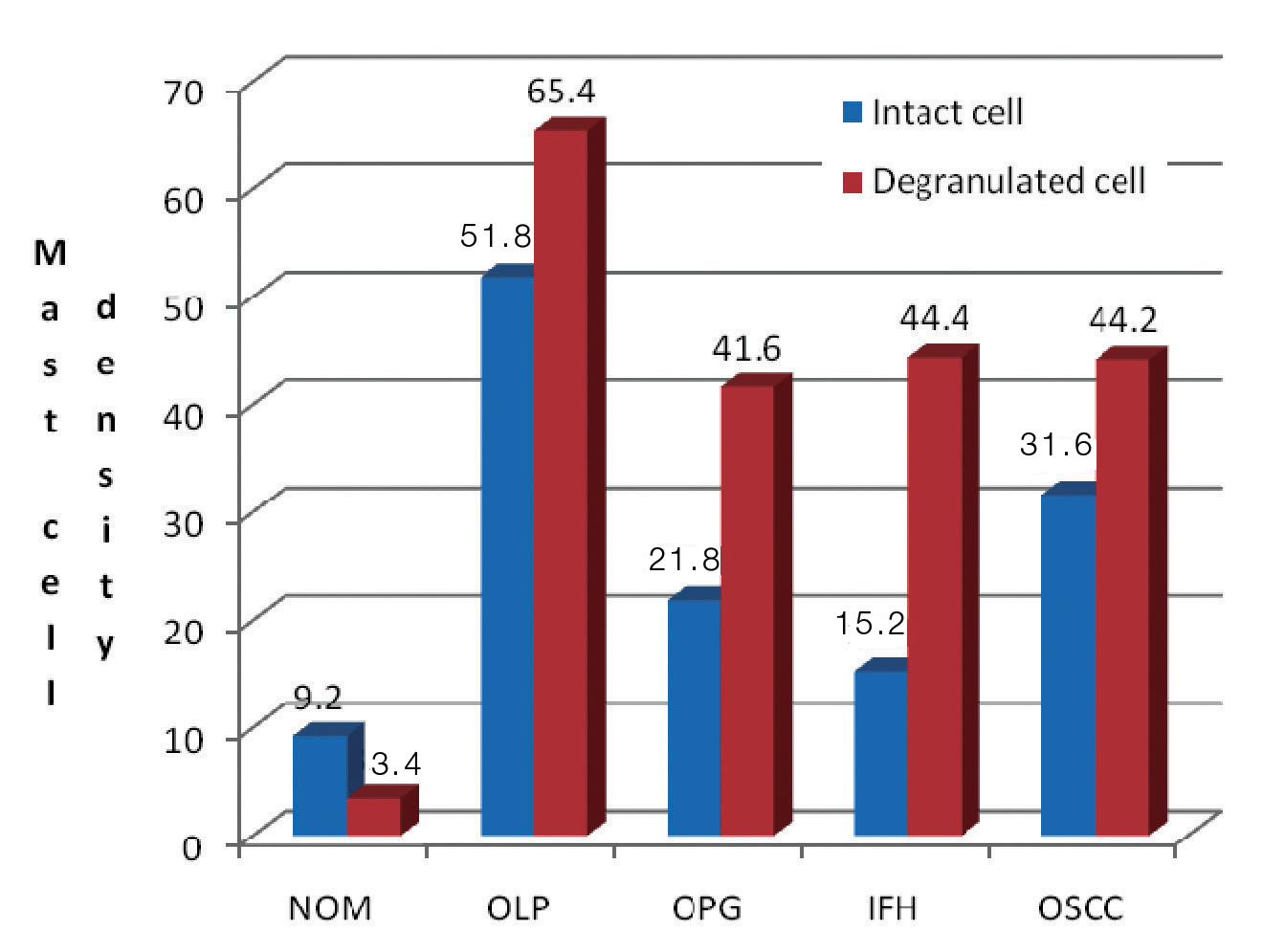
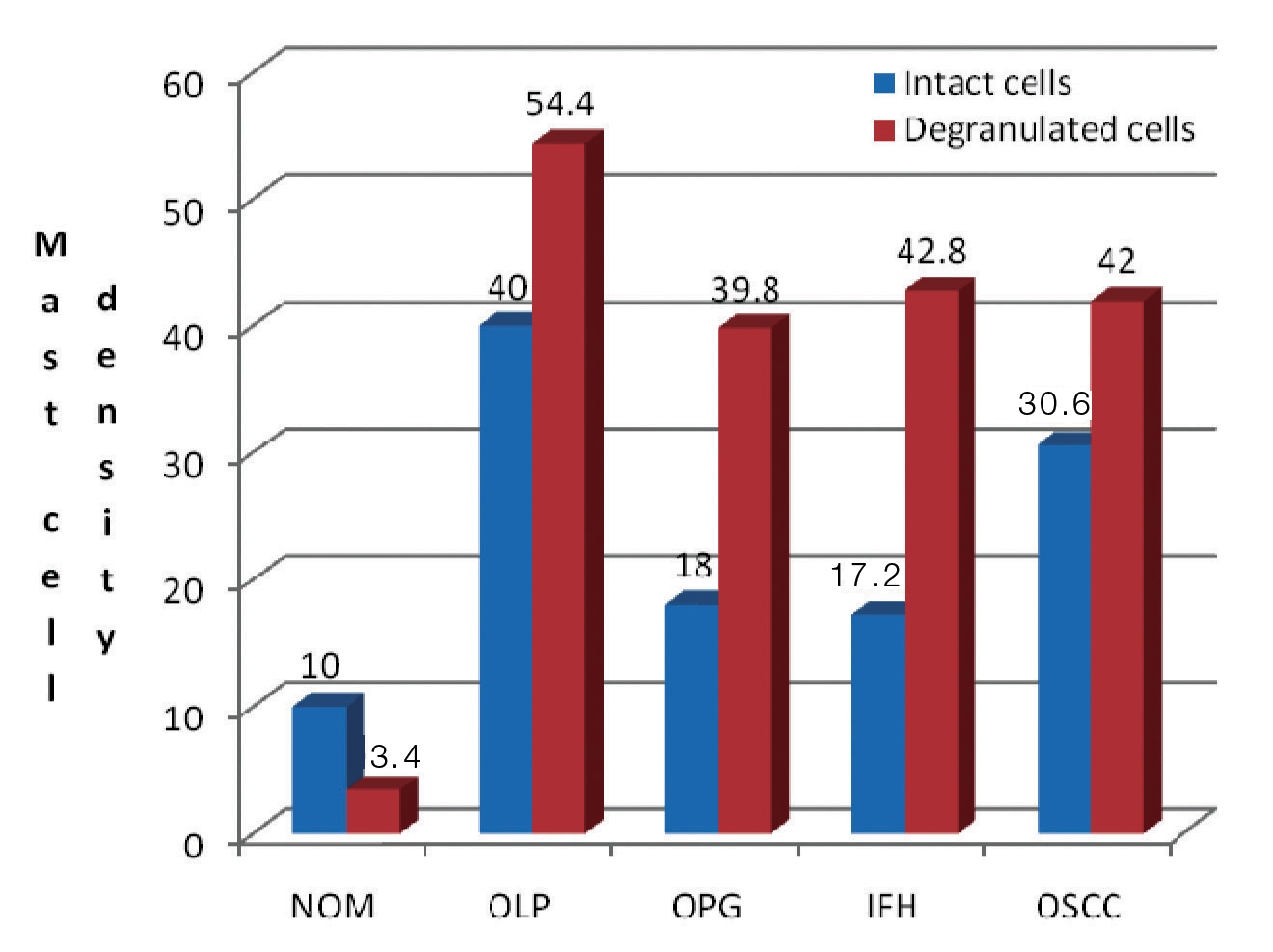
Regardless of the stains used, all four lesions demonstrated an increase in MC count when compared to NOM. With both stains under study, the highest mast cell count (intact and degranulated) was estimated in OLP. With toluidine blue [Table/Fig-3], OLP (65.4±9.71) revealed maximum mean total of degranulated MCs than intact cells followed by IFH (44.4±3.97), OSCC (44.2±1.78) and OPG (41.6±23.04). Similar to toluidine blue, azure A [Table/Fig-4] also demonstrated higher mean total of degranulated MCs in OLP (54.4±20.25) followed by IFH (42.8±3.63), OSCC (42±5.47) and OPG (39.8±27.57). As the results indicate [Table/Fig-5], all four lesions showed an increase in degranulated MCs than intact cells. Comparing both stains the mean total MC count is more in sections stained with toluidine blue than azure A in almost all the lesions under study.
Mast cell density in all lesions understudy stained by toluidine blue.
Mast cell density in all lesions understudy stained by azure A.
Comparison of mast cell count in various lesions under study.
| Lesions | Toluidine blue | Azure A |
|---|
| Intact cell | Degranulated cell | Intact cell | Degranulated cell |
|---|
| NOM | 9.2 ± 2.86 | 3.4 ± 1.14 | 10 ± 2.54 | 3.4 ± 1.14 |
| OLP | 51.8 ± 19.63 | 65.4 ± 9.71 | 40 ± 13.09 | 54.4 ±20.25 |
| OPG | 21.8 ± 11.94 | 41.6 ± 23.04 | 18 ± 13.69 | 39.8 ± 27.57 |
| IFH | 15.2 ± 3.11 | 44.4 ± 3.97 | 17.2 ± 3.11 | 42.8 ± 3.63 |
| OSCC | 31.6 ± 1.34 | 44.2 ± 1.78 | 30.6 ± 3.57 | 42 ± 5.47 |
Discussion
MCs are specialized cells of the immune system which have both pro-inflammatory and anti-inflammatory effects. Following the release of both primary and secondary chemical mediators (degranulation), these cells can exert a direct effect on inflammation and indirectly they may act by recruiting other cell types like lymphocytes to the site. An extensive search of literature revealed that MCs have been studied in OLP, leukoplakia, OPG, peripheral giant cell granuloma, peripheral ossifying fibroma, gingivitis, periodontitis, oral submucous fibrosis and oral cancer [8-10]. However, in most of these studies, MCs were estimated as intact cells rather than counting them separately (intact and degranulated cells).
OLP is an immunologically mediated disease characterized by chronic inflammation. The pathogenesis of OLP has been hypothesized as antigen specific immune mechanism and also by other non specific mechanisms like MC degranulation. MCs release a wide range of pro-inflammtory chemokines like tumour necrosis factor-α (TNFα), tryptase, chymase etc. TNFα causes increased synthesis of matrix metalloproteinases like collagenase, which can induce basement membrane destruction. Chymase a protease released by MCs can activate MMP9 and also lead to basement membrane destruction. Increased expression of adhesion molecules like E-selectin, Intercellular Adhesion Molecule (ICAM) are also generated by TNFα which in turn could probably result in increased leukocytic migration [11]. Histamine, yet another chemical mediator released by MC causes vasopermeability leading to submucosal edema and antigen induced T-cell proliferation. These chemokines released by MCs thus add to the chronicity of this lesion [12,13].
In the present study, maximum amount of degranulated MCs (65.4±9.71 with toluidine blue and 54.4±20.25 with azure A) were noticed in OLP. The results are consistent with studies conducted by Ankle MR et al., Sharma R et al., and Janardhanan M and Ramesh V using toluidine blue stain [8,14,15].
The mean total MC count in OSCC with toluidine blue estimates to 44.2±1.78 degranulated and 31.6±1.34 intact cells while with azure A, degranulated cells accounts for 42±5.47 and intact cells 30.6±3.57. These results were consistent with researches conducted by Ankle MR et al., Anuradha A et al., and Zaidi MA and Mallick AK [8,16,17]. All the cases selected in the current study were well differentiated OSCC and the MCs were mostly seen in the stroma separating the tumour islands. The most important factor assisting in the progression and metastasis of oral cancer is angiogenesis or neovascularisation. In cancer, angiogenic factors are secreted either by the tumour cells or host immunoregulatory cells. The chemical mediators like transforming growth factor α and β, basic fibroblast growth factor, vascular endothelial growth factor, chymase are all angiogenesis promoting factors released by MCs [18]. The tumour released angiogenic factors are capable of further proliferation and migration of MCs [19]. One other property of MC is its phenotypic plasticity which means it is capable of exhibiting variation in mediators with change in the microenvironment [20]. Therefore, the perplexing role of these cells in carcinoma makes them pretty much interesting to researchers. They have also found that MC count tend to decrease with progression of carcinogenesis. This could be due to reduction in immunoregulatory cells due to decreased host immune response. Also, in later stages of carcinogenesis, the tumour cells control angiogenesis rather than factors released by inflammatory cells [21]. However, the process of angiogenesis is not completely understood as it is a complex entity in carcinogenesis.
Inflammation of the oral cavity is a common condition usually presenting clinically as gingivitis, periodontitis, epulis fissuratum etc. IFH is a histopathological terminology usually representing epulis fissuratum or denture related injuries. It is usually caused by chronic irritation of ill fitting dentures. In our study, degranulated MC count was more in IFH when compared to the intact MC count with both toluidine blue and azure A. The increased number of degranulated MCs suggests a possible role of chemical mediators released by the mast cells in IFH. TGF-β and tryptase released by MCs can stimulate the fibroblast growth and the chronicity of this inflammation can lead to fibrosis of the lesion [10]. The presence of MCs was noticed towards the superficial areas of the lesion rather than the fibrotic areas.
The role of MCs in OPG is indeed a less often studied topic. However, a number of studies have been conducted in the past on cutaneous vascular proliferations like haemangioma, angiolipomas, pyogenic granuloma, cherry angiomas etc., [22]. OPG, considered to be a reactive lesion occurs in response to local irritants like calculus, trauma etc. In the present study, the mean total count of MCs in pyogenic granuloma was found to be higher than NOM. Also, the mean total count of degranulated MCs (41.6±23.04 with toluidine blue and 39.8±27.57 with azure A) were more than intact cells (21.8±11.94 with toluidine blue and 18±13.69 with azure A) with both stains under study. Therefore, it is certain that MC could have a possible direct or indirect effect on the development of this lesion. Similar results were found in a study conducted by Kamal R et al., in OPG using toluidine blue stain [9]. They have elucidated that MC degranulation in OPG occurs by immunologic or non immunologic response elicited by local aetiologic factors. This degranulation releases several proangiogenic and angiogenic mediators such as histamine, heparin, chymase, basic fibroblast growth factor, vascular endothelial growth factor, transforming growth factor-beta etc., which in turn bring about the inflammatory and vascular changes in OPG. Also, Iamaroon A suggests that MC have the capacity to stimulate endothelial cells and various other angiogenesis promoting cells like fibroblasts, epithelial cells and macrophages [21]. In our study, MCs in OPG, were seen near the budding capillaries and blood vessels in the mucosa.
Both toluidine blue and azure A are metachromatic stains belonging to the phenothiazine group [6]. But the MC density was more in sections stained with toluidine blue rather than azure A suggesting toluidine blue could be more effective in staining MCs. An extensive literature review of the internet data could not reveal any previous comparative studies between toluidine blue and azure A therefore, our study is one of a kind.
In our study, all four lesions exhibited an increase in mean total MC count when compared to NOM. An increase in MC count indicates either a direct or indirect role of MCs in all these lesions. Also, the interaction of MCs with other immune related cells can be significant to the probability of therapies to targeted MC responses.
Limitation
The study needs to be conducted in a greater number of cases and with other metachromatic stains using different pH levels. This can pave a way to an efficient method of MC staining.
Conclusion
This study concludes that MC density is more in OLP followed by OSCC, IFH and OPG when compared to NOM with both toluidine blue and azure A. Toluidine blue could be more effective than azure A in determining MCs in formalin fixed paraffin embedded tissues.