Materials and Methods
Two hundred and fifty one mother’s admitted for labour at a tertiary care hospital at Kolar, between December 2014 and October 2016, who consented to participate in the study along with their newborns, were included in this cross-sectional observation study. Pregnant women admitted as obstetric emergencies for labour were excluded from the study. Anti-Toxoplasma IgG antibody titres were estimated in the sera of mothers and anti-Toxoplasma IgM antibodies were determined in the cord blood of the newborns. The sample size was calculated taking into consideration the pan India sero-prevalence of 22.4% [9]. The study was approved by the institutional ethics committee and informed consent was obtained from the participating mothers.
Detection of maternal anti-Toxoplasma IgG antibodies
The antibody levels were estimated using a commercial anti-Toxoplasma IgG ELISA kit (NovaTec Immundiagnostica, Germany). A titre of ≥35 International Units/ millilitre (IU/ml) was considered as positive as per the manufacturer’s criteria. The demographic and socio-economic data, educational status, obstetrical history and exposure to risk factors of toxoplasma infection among the participants in the study were recorded on a predesigned proforma. The socio-economic classification was done on the basis of B.G. Prasad socioeconomic scale May 2016 [10].
Detection of anti-Toxoplasma IgM antibodies in cord blood samples
Two hundred and fifty one cord blood samples of the newborns of the above mothers were tested for anti-Toxoplasma IgM antibodies by the anti-Toxoplasma IgM μ capture ELISA (NovaTec Immundiagnostica kit, Germany) as per the manufacturer’s instructions.
Establishing validity of IgM positive reactions
The validity of an IgM positive reaction in the cord blood was evaluated by: a) estimating the IgG antibody levels in the babies’ cord blood, as done for serum samples from mothers, to look for any significant difference in titres between babies’ and that of mothers; and b) by testing the respective mother’s serum sample for anti-Toxoplasma IgM antibodies which could suggest recent infection in the mother. Wherever necessary, the children were followed up by doing a thorough clinical examination and specially looking for any of the clinical features of congenital infections such as hydrocephalus, chorioretinitis and delay in developmental mile stones. In such children, anti-Toxoplasma IgG levels and IgM antibodies were also determined after 6 months of age.
Statistical Analysis
The data is presented as frequencies in tables with percentages. The quantitative measures of central tendency have been expressed as mean±standard deviation. The IgG titers among positive mothers were plotted in a distribution curve. Unpaired t-test was used to analyse the differences in mean age among IgG positive and negative mothers. To analyse the difference in proportions Chi-square test was used. A p-value of <0.05 was considered significant.
Results
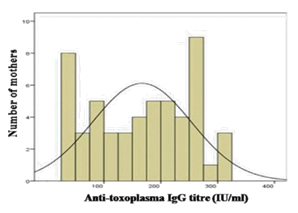
Among the 251 samples tested from mothers, IgG antibodies to T. gondii at titres of ≥ 35 IU/ml were detected in the sera of 53 (21.1%) of them. The titres ranged between 35 IU/ml – 350 IU/ml with a mean ± standard deviation of 167±86 IU/ml. The distribution of titres conformed to normal distribution pattern [Table/Fig-1].
Distribution of anti-Toxoplasma IgG titers among the 53 positive mothers.
The demographic, socio-economic, and behavioural characteristics of mothers along with seroprevalence of Toxoplasma IgG antibodies are presented in [Table/Fig-2]. Majority of the mothers (58 %) were in the first half of third decade of life. Housewives constituted 80 % and most of them (95%) were literate. The population belonged to middle and lower socio-economic classes. A sizeable proportion (43%) belonged to lower socio-economic strata. The study participants in general consumed salad, drank untreated water, and rarely owned cats.
Demographic, socio-economic, and behavioural characteristics of mothers along with seropositivity for Toxoplasma IgG antibodies.
| Characteristics of mothers | Total tested N=251 | No. positive for Taxoplasma IgG antibodies, n(%) | p-value* |
|---|
| Age group |
| 18-20 | 44 | 6(13.6) | 0.143 |
| 21-25 | 145 | 34 (23.4) |
| 26-30 | 53 | 9 (16.9) |
| 31-35 | 9 | 4(44.4) |
| Occupation |
| Housewives | 201 | 44 (21.9) | 0.330 |
| Daily wage manual labourers (Coolie) | 17 | 5 (29.4) |
| Farmers | 8 | 2 (25) |
| Others† | 25 | 2 (8) |
| Education |
| Literate | 238 | 48 (20.2) | 0.116 |
| Illiterate | 13 | 5 (38.5) |
| Socio-economic Status |
| Middle class | 144 | 23 (16) | 0.021 |
| Lower class | 107 | 30 (28) |
| Owning cat |
| Yes | 35 | 7 (20) | 0.862 |
| No | 216 | 46 (21.3) |
| Consumption of Salad |
| Yes | 191 | 37 (19.4) | 0.228 |
| No | 60 | 16 (26.7) |
| Drinking untreated water |
| Yes | 157 | 30 (19.1) | 0.316 |
| No | 94 | 23 (24.5) |
†Tailor, Nurse, Teacher, Engineer, Clerk, Pharmacist
*Chi-square Test; p-value of <0.05 was considered significant
There was no statistically significant difference between the mean age of the seropositive and seronegative mothers (t=1.298 and p=0.195). The prevalence of antibodies among the mothers belonging to lower socio-economic strata was 28% as compared to 16% among those belonging to middle classes (class 3 and 4). This higher prevalence among women of lower socio-economic strata was statistically significant (p=0.021). There was no significant association with seropositivity and occupation, literacy, owning of cats, and consumption of salad, or drinking untreated water [Table/Fig-2]. The obstetrical characteristics of mothers along with seroprevalence of Toxoplasma IgG antibodies are presented in [Table/Fig-3]. Multigravida outnumbered primigravida, majority of them had term gestation and they delivered normally. There was no significant association with seropositivity and gravid status, gestational age, previous history of abortion, and mode of delivery (p =0.231, 0.174, 0.451, 0.724 respectively).
Obstetrical characteristics of mothers along with seropositivity for Toxoplasma IgG antibodies.
| Characteristics ofmothers | Total tested,N=251 | No.positive forToxoplasmaIgG antibodies, n(%) | p-value* |
|---|
| Gravida |
| Primigravida | 113 | 20(17.7) | 0.231 |
| Multigravida | 138 | 33(23.9) |
| Gestational age |
| Term | 230 | 51(22.2) | 0.174 |
| Preterm | 21 | 2(9.5) |
| Previous history of abortion |
| Present | 39 | 10(25.6) | 0.451 |
| Absent | 212 | 43(20.3) |
| Mode of delivery |
| Normal delivery | 175 | 38(21.7) | 0.724 |
| Caesarean section | 76 | 15(19.7) |
*Chi-square Test; p-value of <0.05 was considered significant
Cord blood samples from 5 (2%) of the 251 babies gave a positive anti-Toxoplasma IgM antibody reaction. The Optical Density (OD) values of the positives were just above the cut-off value: they were on an average 0.043±0.015 above the cut-off value of the test in which they were included. IgG antibodies to T. gondii could only be detected in the cord blood sample of 1 of these 5 babies. Anti-Toxoplasma IgM positivity and IgG antibodies in the cord blood of these babies and in the serum samples from their respective mothers is presented in [Table/Fig-4].
Anti-Toxoplasma IgM and IgG antibodies in the cord blood of babies and sera from their respective mothers.
| Case | IgM in Baby’s cord blood | IgG in Baby’s cord blood | IgG in Mother’s serum | IgM in Mother’s serum | Inference |
|---|
| 1 | + | - | - | - | False positive |
| 2 | + | - | - | - | False positive |
| 3 | + | - | - | - | False positive |
| 4 | + | - | - | - | False positive |
| 5-1 | +- | +- | +N.D | -N.D | False positive |
| 5-2* |
*Venous blood sample of the baby collected at 11 months on follow up, N.D = Not Done
The IgM positivity in the cord blood samples of the newborns were considered as false positive reaction in 4 cases, because there was no evidence of maternal infection at all: The serum samples simultaneously collected from their mothers lacked IgG and IgM anti-Toxoplasma antibodies. Though both, the cord blood samples from the baby and mother’s serum sample had IgG antibodies in one case, there was no four-fold difference in titres between them (case 5: [Table/Fig-4]) and on follow up of this child at 11 months of age neither IgG nor IgM antibodies to T. gondii could be detected in the serum sample; there were no clinical features of congenital toxoplasmosis either.
Discussion
We report here a seroprevalence of 21.1% for anti-Toxoplasma IgG antibodies among the rural pregnant women tested from Kolar region, southern Karnataka. Thus, 79% of pregnant women who lacked anti-Toxoplasma antibodies are at the risk of acquiring primary infection during pregnancy. Seropositivity rates comparable to that found in our study, have also been reported by earlier studies from India [3,9]. However, the seroprevalence rates do not seem to be uniform throughout the country. It is reported to be as high as 77% in Kumaon region of Himalayas, 48% in Northeast India, and 37% in Coastal Karnataka [4,9,11].
The prevalence of Toxoplasma antibodies may vary in different populations depending upon climatic and socio-economic conditions, and behavioural patterns [9]. In our study we observed that there was a significant association between seropositivity and lower socioeconomic strata. Similar observation has been made in an earlier study from Assam [12].
We observed IgM positivity in 5 (2%) of cord blood samples tested, but their validity could not be established; they were considered as false positives. Such false positive reactions in ELISA for anti-Toxoplasma IgM antibodies are well documented [13,14]. We emphasize that one should evince care in reporting IgM positive ELISA reactions for the diagnosis of toxoplasmosis.
One of the mothers in our series had anti-Toxoplasma IgG antibodies in her serum without the presence of anti-Toxoplasma IgM antibodies, indicative of infection in the past. However, considering the possibility of latent infection, we followed up her baby whose cord blood gave positive reaction for anti-Toxoplasma IgM and IgG antibodies. On follow up, we could not find any clinical or serological evidence for vertical transmission of toxoplasmosis. Though vertical transmission of toxoplasmosis has been documented in India, to the best of our knowledge, there are no studies on vertical transmission of toxoplasmosis involving a series of mother-baby pairs. In this direction, our study may be considered as a pilot study. As the vertical transmission of toxoplasmosis is known to be directly proportional to the prevalence of antibodies among women in the child bearing age, studies similar to ours need to be undertaken at least in high prevalence areas of the country [11].
Recently a higher frequency of vertical transmission of toxoplasmosis by using polymerase chain reaction for detection of T. gondii DNA in umbilical cord tissue samples of neonates has been reported from Libya [15]. Such studies based on molecular diagnosis along with serological studies may contribute towards more accurate estimations of vertical transmission in the population.
Limitation
We could not detect any vertical transmission of toxoplasmosis among the 251 cord blood samples of newborns tested; but a false positive IgM reaction was encountered in 5(2%) of the samples tested. If we had detected even 1 case of vertical transmission among 251 newborns, then the vertical transmission rate would have been 0.4 %. Thus the vertical transmission rate in Kolar region appears to be less than 0.4 %. Secondly, we have tested the serum samples from mothers and cord blood samples from newborns for anti-Toxoplasma antibodies of IgG and IgM classes only. Inclusion of tests to detect anti - Toxoplasma IgA and IgE class of antibodies, in addition, would have increased the sensitivity and specificity of detection of recent Toxoplasma infections in mothers and vertical infection in newborns.
Conclusion
Our data shows that only about one fifth of pregnant mothers from Kolar region of Karnataka are naturally immune to toxoplasmosis and a large proportion, nearly 80% women are at risk of toxoplasmosis during pregnancy. In this context it becomes necessary to screen pregnant women routinely to detect recent toxoplasma infections. It is also essential to check the newborns for evidence of congenital toxoplasmosis. These efforts would pave way for prevention of infections during pregnancy and treatment of vertical infections.
†Tailor, Nurse, Teacher, Engineer, Clerk, Pharmacist
*Chi-square Test; p-value of <0.05 was considered significant
*Chi-square Test; p-value of <0.05 was considered significant
*Venous blood sample of the baby collected at 11 months on follow up, N.D = Not Done