Interesting Cystic Lesions of Pancreas in Septuagenarians
Biggs Saravanan Ramachandran1, Maria Jacob2
1 Consultant, Department of Gastroenterology and Interventional Endoscopy, Bharath Hospital, Kottayam, Kerala, India.
2 Registrar, Department of Gastroenterology and Interventional Endoscopy, Bharath Hospital, Kottayam, Kerala, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Maria Jacob, Department of Gastroenterology, Bharath Hospital, Kottayam-686001, Kerala, India.
E-mail: theeditor13@gmail.com
Intraductal papillary mucinous neoplasms are rare pancreatic tumours which can mimic more common diagnoses like pancreatic pseudocysts or pancreatic mass. Here we describe two of our cases who presented with abdominal pain and weight loss and were diagnosed to have Intraductal Papillary Mucinous Neoplasm (IPMN) after Endoscopic Ultrasound-guided Fine Needle Aspiration (EUS FNA) which was crucial in clinching the diagnosis.
Endoscopic Ultrasound-guided Fine Needle Aspiration, Intraductal papillary mucinous neoplasm, Pancreatic cysts
Case Report
We present two histologically diagnosed cases of IPMN who presented to us with chronic abdominal pain and showed features of a pancreatic mass on imaging. Both the cases were diagnosed as IPMN within the span of one year.
Case 1
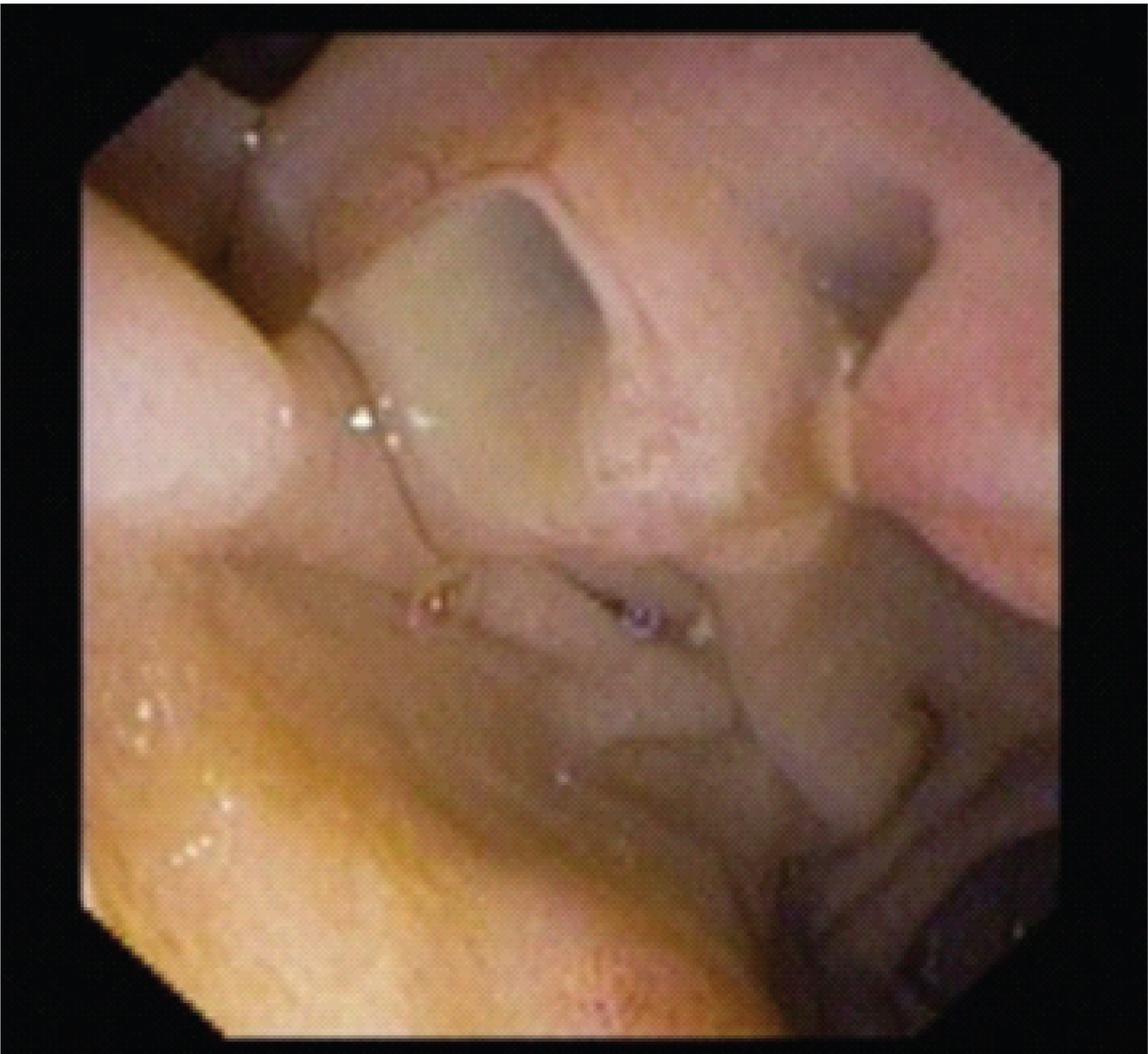
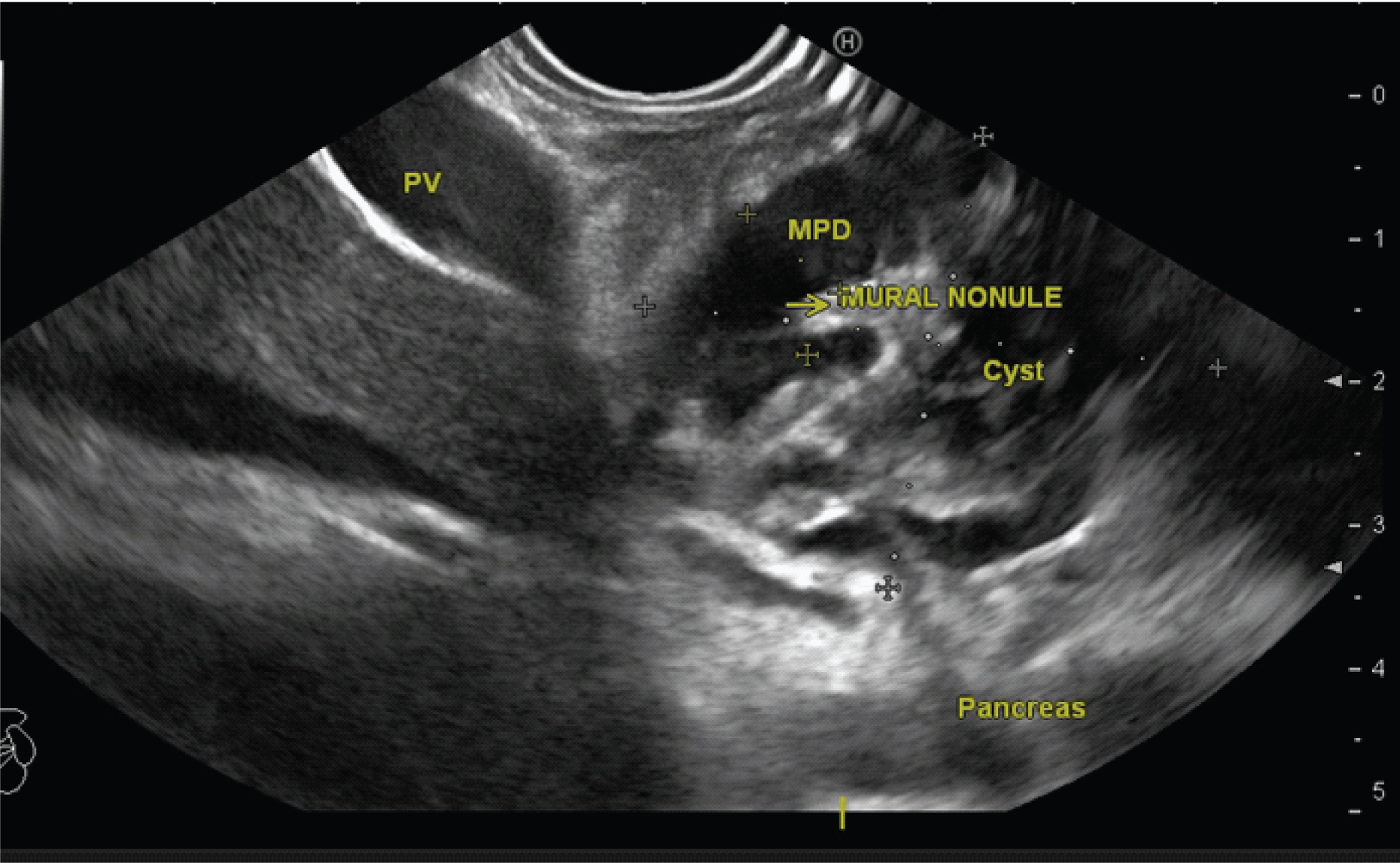
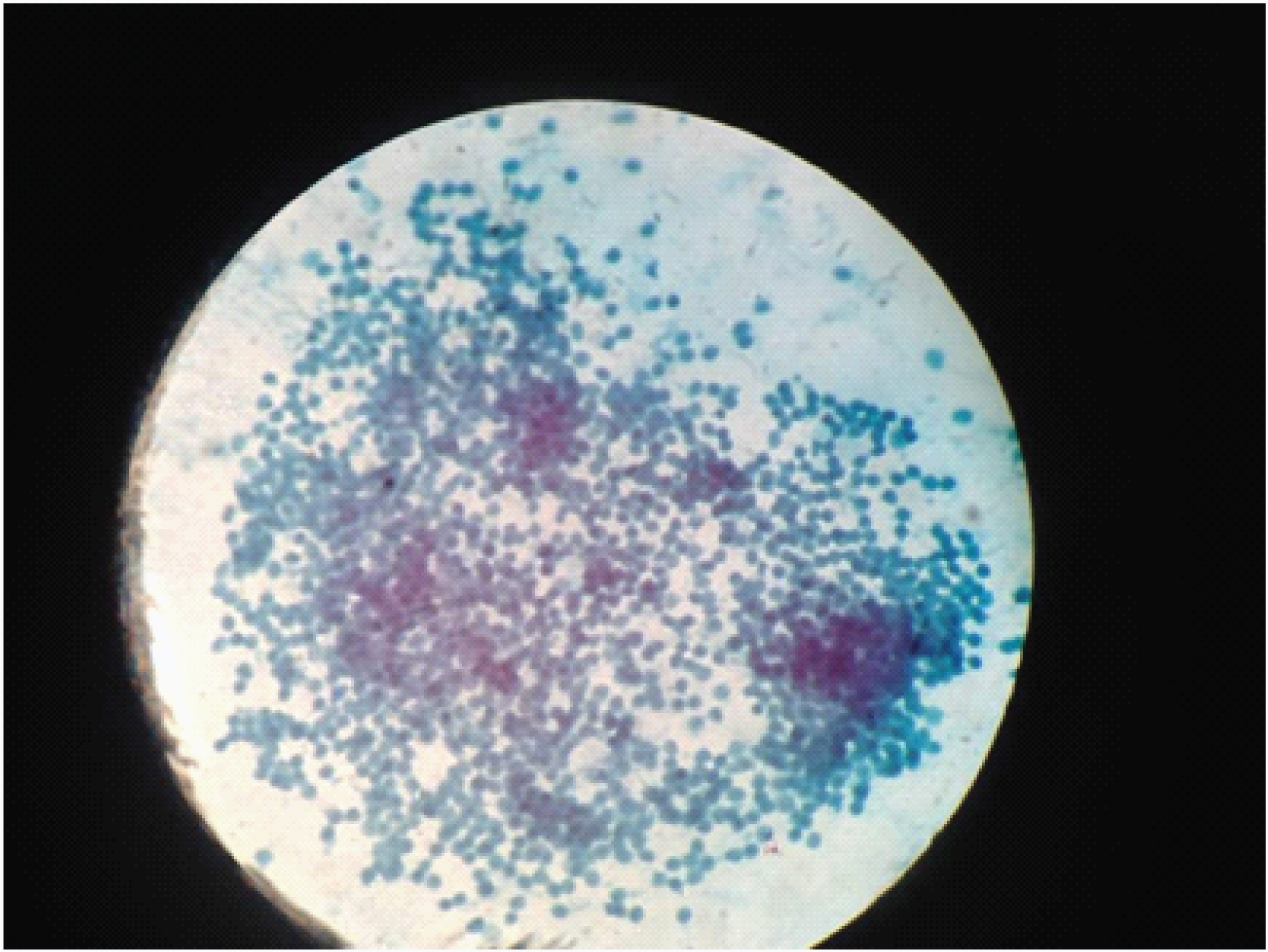
Our first case was that of a 74-year-old female patient, a diabetic who presented with complaints of chronic epigastric pain and a history of acute weight loss. Basic haemogram was found to be normal. Ultrasonography showed a mass in the pancreatic head (5.3×4 cm) with cysts in both head and uncinate process. Main pancreatic duct was found to be dilated. Further investigation in the form of a Contrast Enhanced Computed Tomography (CECT) scan showed a pancreatic head mass (4×3 cm) with cysts in the pancreatic head and uncinate process, with heterogenous enhancement, associated with severe dilatation of main pancreatic duct (16 mm) and its branches. Side viewing scopy showed fish mouth appearance of the ampulla of Vater with mucus extrusion [Table/Fig-1]. Endoscopic Ultrasonography was also done which showed the presence of a large cystic lesion (40×32 mm) with multiple septae and mural nodules, with dilatation of main pancreatic duct and its side branches, involving head and uncinate process [Table/Fig-2]. Fine Needle Aspiration (FNA) of the cyst and mural nodules was done. Cytological evaluation showed epithelial cuboidal cells with uniform round nuclei, vacuolated cytoplasm, anisopoikilocytosis and granular chromatin in a mucinous background suggestive of a mucinous neoplasm [Table/Fig-3].
Side viewing scopy showing fish mouth ampullary opening with extrusion of mucus.
Large cyst involving head and uncinate process with Main Pancreatic Duct dilatation (MPD) and mural nodules inside. Pancreas- pancreatic parenchyma, PV- portal vein, MPD – main pancreatic duct, yellow arrow- mural nodule.
EUS FNA showing epithelial cuboidal cells with uniform round nuclei, vacuolated cytoplasm and granular chromatin in a mucinous background.
Case 2
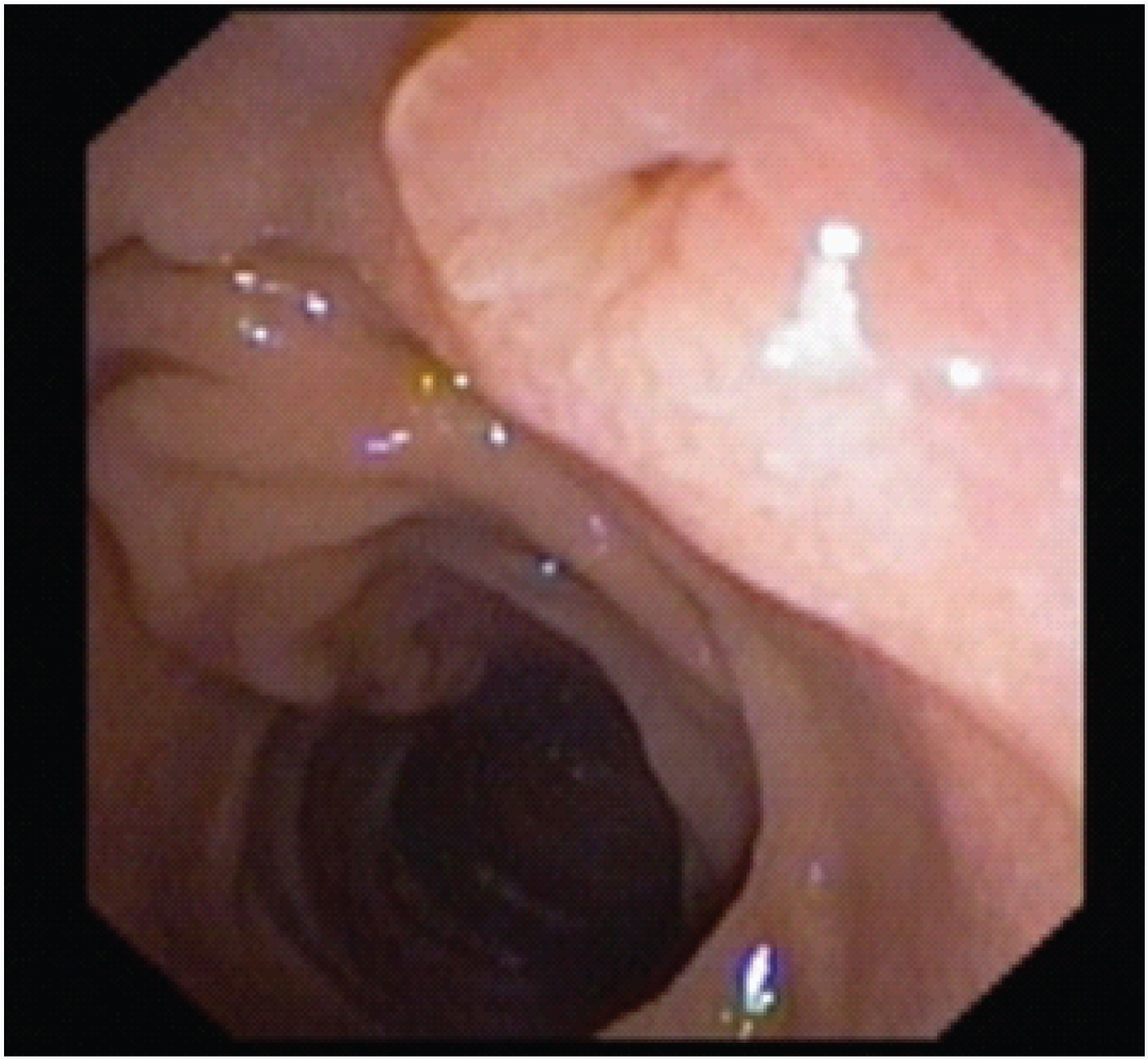
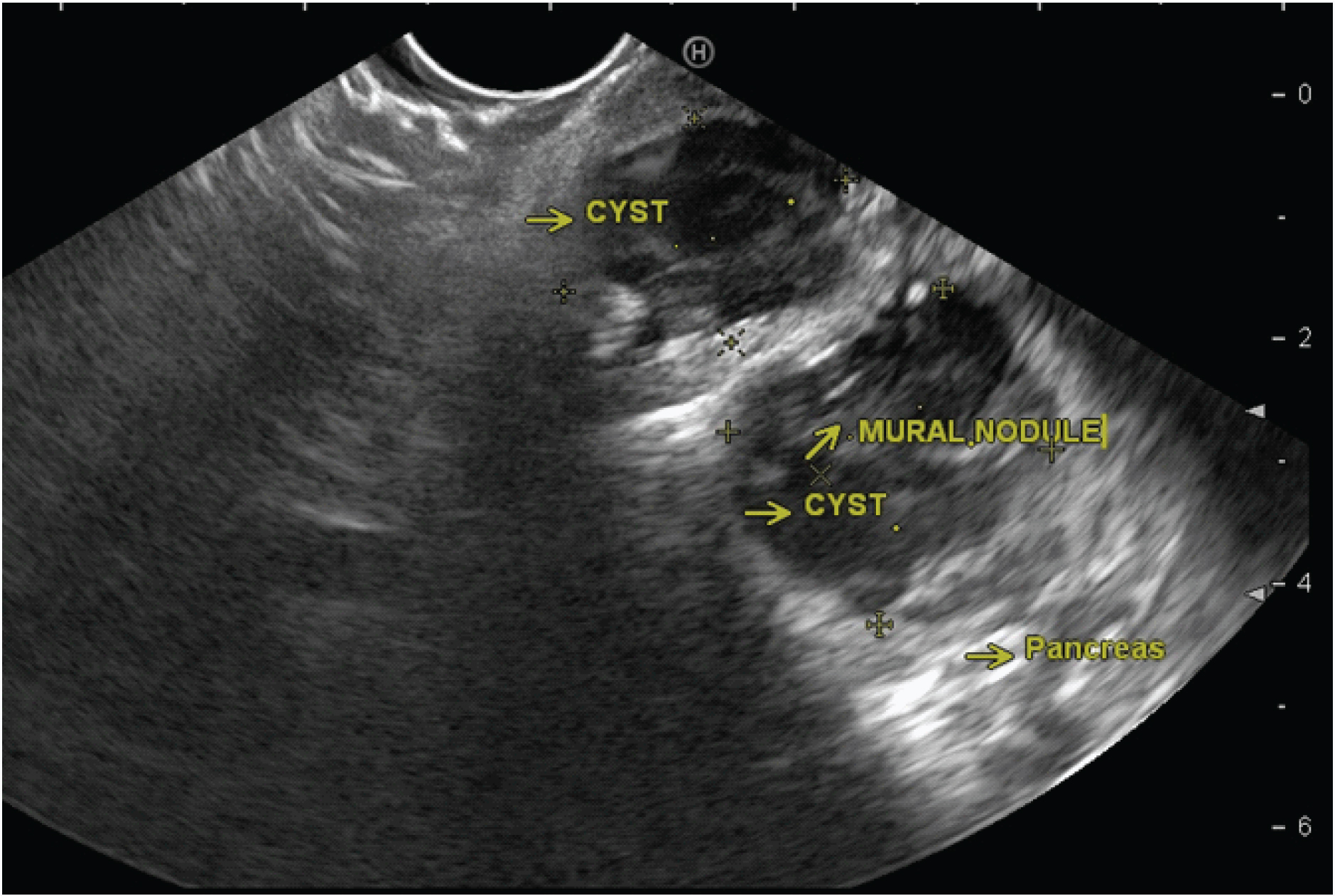
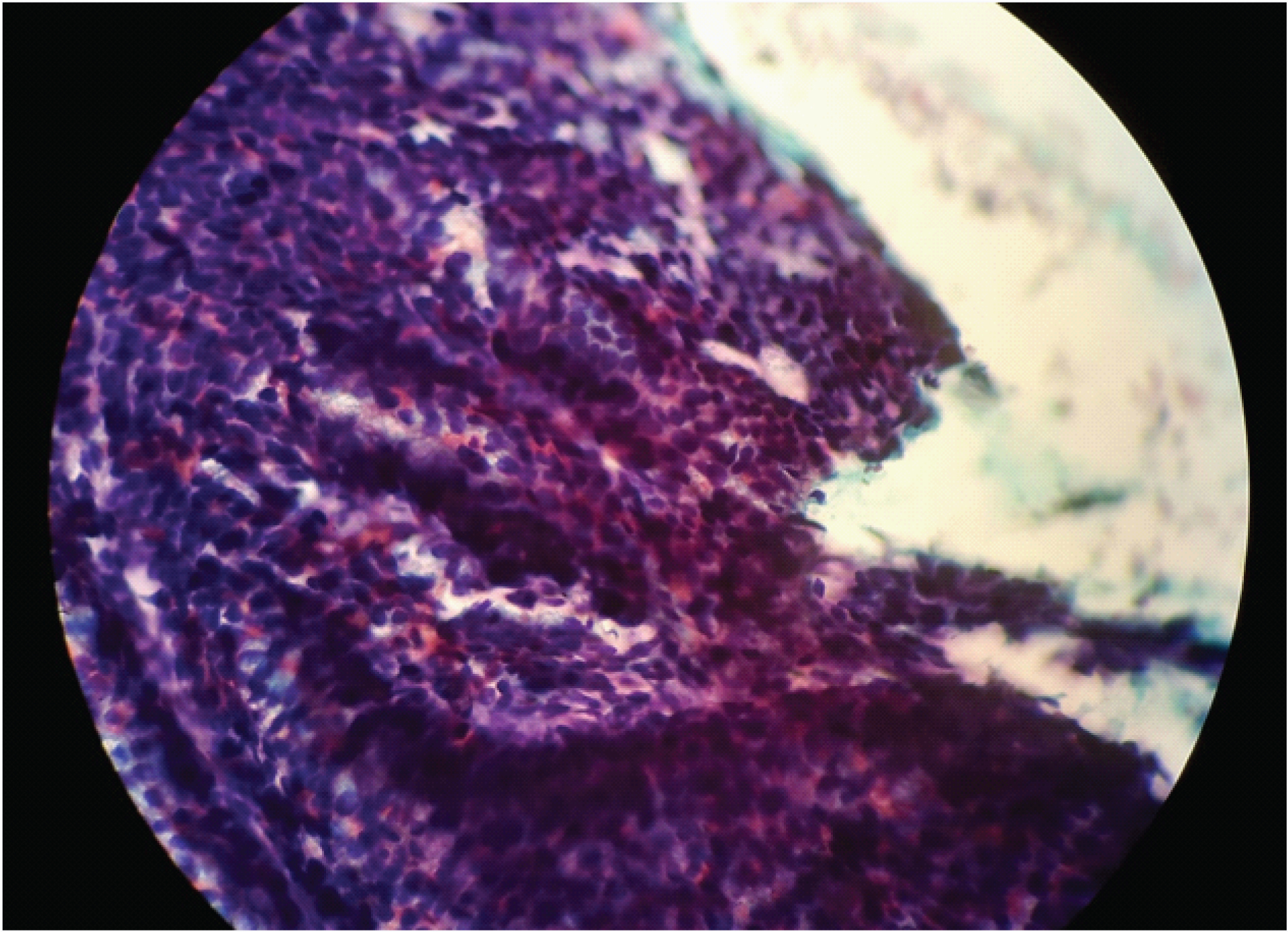
The second case was a 75-year-old diabetic female who presented with complaints of chronic upper abdominal pain associated with a history of loss of appetite and weight. In her case also, haemogram was found to be normal. Computed tomography scan showed an atrophic pancreas with a tubular cystic area (4.3 × 2.2 × 2.8 cm) in the head region with peripheral calcifications. It was continuous with the main pancreatic duct which was seen to be dilated (12 mm in diameter). These features were suggestive of chronic pancreatitis with pseudocyst formation. Here too, a side viewing scopy was done, which showed fish mouth appearance of the ampulla of Vater with mucus extrusion [Table/Fig-4]. Endoscopic Ultrasonography showed a cystic neoplasm (40 × 20 mm) of pancreas involving the head and uncinate process. Main pancreatic duct and its side branches showed upstream dilatation and the presence of multiple mural nodules [Table/Fig-5]. Pancreatic body and tail were found to have atrophied. Fine Needle Aspiration of the cyst was done and sent for analysis. Cytological evaluation showed papillary fragments with honey-combed sheets of epithelial cells with uniform nuclei [Table/Fig-6]. There was no significant nuclear pleomorphism, nuclear anaplasia or mitosis identified. All the microscopic features were suggestive of IPMN.
Side viewing scopy showing fish mouth ampullary opening with extrusion of mucus.
Large multiloculated cyst with mural nodules. Pancreas-pancreatic parenchyma, Cyst- pancreatic head region cyst. Yellow arrow- mural nodules.
EUS FNA showing papillary fragments with honey-combed sheets of epithelial cells of uniform nuclei. There was no significant nuclear pleomorphism, nuclear anaplasia or mitosis.
Both the cases were diagnosed as IPMN and referred for surgical gastroenterology management.
Discussion
Intraductal Papillary Mucinous Neoplasm is a rare intraductal epithelial tumour which is grossly visible (> 10 mm) in size. It constitutes about 10-15% of the total cystic tumours of the pancreas and 0.5% of the total pancreatic tumours. It is more frequently seen in older patients (50-60 years) and has a male predilection [1]. A study conducted at John Hopkins Hospital on a group of asymptomatic individuals, came to the conclusion that IPMN of pancreas is becoming fairly common, particularly in the elderly [2]. They are intraductal neoplasms characterized by an over-production of mucinous secretion by a proliferative epithelium with papillary growth. They are important because some of them have the potential to progress towards invasive carcinoma of the pancreas if left untreated [2].
Based on the site of cyst formation, it is classified into three groups: 1) Main Pancreatic Duct IPMN (MD – IPMN) with > 70% malignancy potential; 2) Branched duct IPMN (BD – IPMN) with a lower risk for developing malignancy (20% in 10 years time); 3) Both main and branched duct involving type of IPMN [1]. Main pancreatic duct is the most common site of IPMN. Grossly, these form long thin structures projecting into the duct, while microscopically, they are composed of columnar tumour cells containing mucin. Pathologically, they are divided into two broad groups:1) those associated with invasive carcinoma; and 2) those not associated with invasive carcinoma. This classification has a critical prognostic significance. Based on the site of the tumour, they are classified into main duct type and branched duct type IPMN. This is important as branched duct IPMNs are less aggressive than the main duct type [3,4].
Most common symptoms associated with IPMN are abdominal pain (most common symptom) with 55% incidence [1], nausea and vomiting. Most common signs are jaundice, weight loss and features of acute pancreatitis [5] and a recent onset of diabetes mellitus. Laboratory features are usually normal. There is an abnormal increase in the levels of amylase which helps in differentiating IPMN from Mucinous Cystic Neoplasm (MCN) (which shows low levels of serum amylase). If there is a gross increase in amylase levels, it indicates invasiveness. Detection and diagnosis of IPMN is hence accidental, following an unrelated imaging of the abdomen, due to its nonspecific signs and symptoms.
Diagnostic confirmation is made by either a Computed Tomography scan, Endoscopic Ultrasonography or a Magnetic Resonance Cholangio-Pancreatography, which will show dilatation of the main pancreatic duct and/or one of its branches. Fine Needle Aspiration during EUS and its histopathological evaluation can clinch the diagnosis.
Characterization of pancreatic IPMNs, especially the differentiation between benign and malignant lesions is limited in CT and MRI scans. Herein comes the importance of the high resolution, close target-proximity imaging by EUS and its ability to take samples. IPMNs are often macrocystic lesions with parenchymal changes (due to ductal obstruction) and communication with the pancreatic duct [6]. “EUS findings in IPMN include segmental or diffuse, moderate to marked dilatation of main pancreatic duct. Differentiation between MD – IPMN and BD – IPMN is also possible by EUS. Dilatation of the main pancreatic duct > 1cm indicates MD – IPMN (Dilatation > 5mm, if no other causes of obstruction are present [5]).” Presence of a cyst communicating with the pancreatic duct without MD dilatation or with MD diameter < 6mm suggests BD – IPMN. The presence of mural nodules strongly points to increased risk of malignancy and EUS is the most sensitive imaging for the detection of mural nodules [7].
Surgical resection is an important modality of treatment in case of main duct IPMN. Depending on the site of the lesion, we can do either a distal pancreatectomy (in case of a tail lesion) or a Whipple’s procedure (in case of a head/uncinate lesion). Rarely a total pancreatectomy is indicated in cases where IPMN involves the entire length of the pancreas.
In case of an asymptomatic branched duct IPMN with: a) a lesion of size less than 3 cm; b) no associated dilatation of main pancreatic duct; and c) no solid mass/ mural nodule, we can safely follow up the patients without surgery. If the above criteria are not fulfilled, then we can proceed with the surgical option [8,9].
Even after surgical resection, regular follow up is required as these patients are at risk to develop a second lesion [10,11]. Patients with IPMN have shown an increased risk of developing colonic and rectal tumours, in comparison to the normal population [11]. Hence regular colonoscopic follow-up is also recommended for these patients.
In both our cases, patient presented with chronic abdominal pain along with a history of weight loss. Both were elderly and had a normal haemogram. Pancreatic lesion detected by a CT imaging of the abdomen was confirmed by EUS and the diagnosis was clinched by the FNA cytology of the cyst fluid which showed microscopic features consistent with IPMN. The option of surgical resection was given to both the patients who were then referred to surgical gastroenterology.
Conclusion
IPMNs are rare pancreatic tumours which present as pancreatic masses or cysts, thus mimicking more common diagnoses like pancreatic pseudocyst or pancreatic adenocarcinoma. EUS, especially when combined with FNA has the discriminative advantage of differentiating IPMN from other masses and cystic lesions of the pancreas.
[1]. Podolsky DK, Camilleri M, Fitz JG, Kalloo AN, Shanahan F, Wang TC, Yamada’s textbook of gastroenterology 2016 Vol 26th edHoboken (NJ)J.Wiley & Sons:1751-1754. [Google Scholar]
[2]. Laffan TA, Horton KM, Klein AP, Fishman EK, Johnson PT, Hruban RH, Prevelance of unsuspected pancreatic cysts on MDCTAJR Am J Roentgenol 2008 191(3):802-07. [Google Scholar]
[3]. Terris B, Ponsot P, Paye F, Hammel PR, Sauvanet A, Molas G, Intraductal papillary mucinous tumours of the pancreas confined to secondary ducts show less aggressive pathologic features as compared with those involving the main pancreatic ductAm J Surg Pathol 2000 24(10):1372-77. [Google Scholar]
[4]. Bernard P, Scoazec JY, Joubert M, Kahn X, Le Borgne J, Berger F, Intraductal papillary-mucinous tumours of the pancreas: predictive criteria of malignancy according to pathological examination of 53 casesArch Surg 2002 137(11):1274-78. [Google Scholar]
[5]. Tanaka M, Chari S, Adsay NV, Fernandez-del Castillo C, Falconi M, Shimizu M, International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreasPancreatology 2006 6(1-2):17-32. [Google Scholar]
[6]. Efthymiou A, Podas T, Zacharakis E, Endoscopic ultrasound in the diagnosis of pancreatic intraductal papillary mucinous neoplasmsWorld J Gastroenterol 2014 20(24):7785-93. [Google Scholar]
[7]. Lim LG, Itoi T, Lim WC, Mesenas SJ, Seo DW, Tan J, Current status on the diagnosis and management of pancreatic cysts in the Asia – Pacific region: role of endoscopic ultrasoundJ Gastroenterol Hepatol 2011 26(12):1702-08. [Google Scholar]
[8]. Lee CJ, Scheiman J, Anderson MA, Hines OJ, Reber HA, Farrell J, Risk of malignancy in resected cystic tumours of the pancreas < or =3 cm in size: is it safe to observe asymptomatic patients? A multi-institutional reportJ Gastrointest Surg 2008 12(2):234-42. [Google Scholar]
[9]. Rodriguez JR, Salvia R, Crippa S, Warshaw AL, Bassi C, Falconi M, Branch-duct intraductal papillary mucinous neoplasms: observations in 145 patients who underwent resectionGastroenterology 2007 133(1):72-310. [Google Scholar]
[10]. Zamora C, Sahel J, Cantu DG, Heyries L, Bernard JP, Bastid C, Intraductal papillary or mucinous tumours (IPMT) of the pancreas: report of a case series and review of the literatureAm J Gastroenterol 2001 96(5):1441-47. [Google Scholar]
[11]. Sugiyama M, Atomi Y, Extrapancreatic neoplasms occur with unusual frequency in patients with intraductal papillary mucinous tumours of the pancreasAm J Gastroenterol 1999 94(2):470-73. [Google Scholar]