Most of the newborns in the NICU (Neonatal Intensive Care Unit) require continuous or intermittent intravenous therapy. The administration of intermittent medication such as antibiotics, antifungal, antiepileptic or blood products is performed using, an immediately accessible IntraVenous (IV) route i.e., a Peripheral Intravenous Lock (PIVL) which is an IV cannula capped with a needleless connection. In some cases, when the patient requires both continuous and intermittent intravenous therapy, an additional PIVL may be used for the intermittent intravenous drug to avoid the interruption of continuous intravenous medication therapy resulting in multiple catheters.
Patency of this cannula is an important issue and PIV catheters are often left in situ in neonates until any complications arise [1]. It’s technically challenging to change the catheter site at predetermined timings and repeated attempts may break the skin barrier and predispose to infection by commensals in the skin which may result in septicemia and can be life threatening. Frequent insertions of a new PIVL is a painful experience for the neonates and tedious, stressful procedure for nurses moreover limited useful veins are available for cannulation in this patient group. Therefore, methods that can prolong the patency of PIV catheters are beneficial in neonates. To administer medications and prevent a potential fluid imbalance, to obtain intermittent access, PIVL in many NICUs are often kept patent by flushing at regular intervals with Heparinized Saline (HS) solutions. HS solutions have been provided in varying concentrations indifferent institutions [2-6]. It has been suggested that heparin is unnecessary to maintain patency, as it can be attributed to the positive pressure in the closing of the IV line [7].
Studies done both in adults and children concluded that a normal saline (NS) flush works equally effective as HS flushes [8-12]. The use of NS flush is a common practice in adult and adolescent patients [11,13]. However, neonates show both sensitivity and resistance to heparin [14] and the cannula used in neonates is smaller than those used in adults.
Though, the common belief among healthcare providers is that small dosage of heparin in the flushing solutions are relatively harmless [11], the decision on whether heparin should be used or not in this group of patients, is clinically important due to the heparin associated risk. Although neonates, have a higher propensity to develop intracranial haemorrhage [15], heparin use can be linked with the occurrence of intraventricular haemorrhage among very low–birth weight babies [16]. It can also cause cerebral haemorrhage in premature infants [17] and is also linked with thrombocytopenia in neonates [16,18-20]. Other concerns regarding heparin use include increased labor and equipment costs to prepare heparinized flush solutions [4,9,11], increased manipulation with the potential for contamination of intravenous sites [21,22], and an increased risk of needle sticks to nurses [22], the possibility of drug error or drug incompatibility [9]. For all of these reasons, it may be safer to use NS as the flush solution. Even if HS flush significantly prolongs the lifespan of intravenous locks [23] compared to NS flush, it is beneficial to neonates as it can decrease the number of intravenous catheter insertions, especially in preterm infants [24].
Several studies have analysed the patency of a PIVL flushed with heparin versus saline in different age groups. But results in neonates are conflicting. Majority of the studies showed no differences between heparin (with concentrations of 1–10 units/mL) versus saline on the patency of PIV catheters [24-28]. Some studies suggested that heparin solution is more effective than saline [29,30].
A meta-analysis (Shah P et al.,) included 10 randomized or quasi-randomized controlled trials [30] and a review (Trautmann M et al.,) concluded that a recommendation for heparin use in neonates with PIVL could not be made due to clinical heterogeneity and diversity in outcome parameters [31]. They concluded a lack of blinding in interventions and allocation; non-uniformity in the dosage and the administration method; the frequency of flushing was not always mentioned. Several studies used different brands and/or size of catheters [30,32]. In these studies, there is no clear recommendation for flush solutions giving a prolonged patency of a PIVL.
The neonatal PIVL management practices vary between hospitals and are based on individual knowledge and practice. There are no clinical practice standards for using a PIVL flush solution in neonates [33]. The study is proposed to address several gaps found in previous studies.
The aim is to assess and compare the efficacy and safety of HS and NS flushes in maintaining the patency of intermittent peripheral IV 24 Gauze catheters in neonates requiring intensive care and to assess the impact of gender, gestational age and body weight on patency of PIVLs.
Materials and Methods
Study Design and Subjects
A prospective study was conducted for a period of 12 months from October 2014 to September 2015 at a 32 bedded NICU of a south Indian tertiary care hospital. The authors designed the study, and it was approved by the Hospital Institutional Ethics committee. Neonates requiring PIVL for medications such as antibiotics, anti-epileptics, antifungals during their hospital stay and weigh 1.5 kg or more and has the gestational age of 34 weeks or greater at enrollment, were included in the study with available written consents from a parent/guardian. Neonates with multiple IV lines/continuous IV fluids/central catheters/umbilical line/diagnosis of disseminated intravascular coagulopathy/thrombocytopenia/haemorrhage/anti-coagulation treatment; neonates who were the recipient of surgery recently /critically ill and who had unwilling parents/guardian were excluded. In our study, a concentration of 1 unit/ml Heparinized Saline (0.1 ml of heparin diluted in 100 ml of Normal saline) and Standard Normal saline IP 0.9% solution was the control and intervention respectively.
Prior to the initiation of the study, nurses involved received training and hands-on practice on the standard method of flushing a PIVL, identifying IV complications, criteria for discontinuing IV catheters and on documentation of all these steps. A 1 ml of flushing solution was flushed into IV line, before and after drug administration and was kept locked inside the catheter. The frequency of flushing was every 8th,12th and 24th hourly as per to medication administration requirements. Preferred sites were dorsum of arm/hand/feet, elbow, ankle joint, and the great saphenous vein of feet.
Sample Size
Sample size for the study was calculated based on the ‘sample size calculation formula for comparing two means’. Sample size required for having 80% power and 95% confidence was approximately 57 patients in each group. Total 120 patients (60 in each group) were to be selected to compensate the probable dropouts.
Randomization and Blinding
Eligible neonates were randomly assigned to FLUSH ‘A’ or FLUSH ‘B’ by the primary investigator using the randomization table, based on Permuted Block Randomization. Specific compounding nurses were assigned to prepare and label the solutions in an aseptic unit on a daily basis. The investigators and nursing staffs involved in the catheter insertion team, baby care, and assessment of the catheter sites were blinded on the group assignment till the end of the study.
Data Collection and Analysis
Subject demographics, lab data like platelet counts and blood culture results, medications administered, the frequency of flushing, time of insertion and removal of the catheter, the reason(s) for discontinuation of each PIVL were collected from various medical records. These details along with solution code, catheter site, calculated patency of each catheter in hours, and the numbers of catheters used for each baby were documented in case record form.
Patient demographic characteristics like gender, gestational age, body weight were used to analyse the effect of these factors on patency of catheters. The mean number of catheters removed due to non-elective reasons in both the groups was calculated to evaluate the effectiveness of flushing solutions. To evaluate the safety, the reason for catheter removal was noted and platelet nadir was used to assess the thrombocytopenia.
Statistical Analysis
Data was analysed using SPSS15. Demographic data were presented as descriptive statistics. Categorical data were described with frequencies and percentages. Poisson regression was used to assess the effectiveness of flush solutions by comparing the mean number of catheters removed due to non-elective reasons in both the groups for variables of interest. A p-value ≤0.05 was considered as statistically significant. Pearson Chi-square test and stratified analysis were conducted to study the frequency of thrombocytopenia.
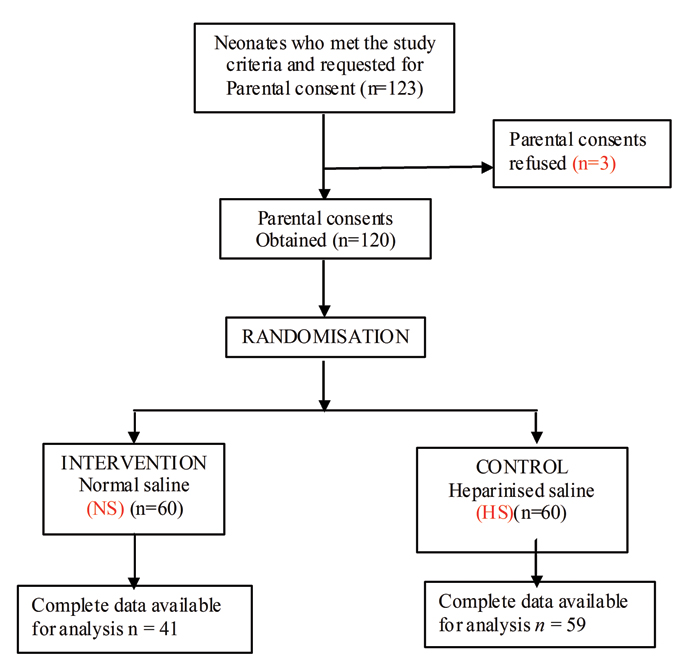
Procedure for selection of patients is represented in [Table/Fig-1] the Consort Flow Diagram. It shows neonates who met the study criteria were 123 during the study period. Among them three parents of neonates refused to give the consent. Total of 120 neonates selected for the study and randomized into 60 neonates of two groups, intervention (NS) and control group (HS). On follow up complete data of 41 neonates in intervention group and 59 neonates of control group was available. These data were used for further analysis.
Results
Data from 100 neonates was available at the end of the study 59 in HS group and 41 in NS group as data of 20 neonates was incomplete. This data was used to evaluate the study objectives.
Demographic Characteristics of the Study Population
The mean gestational age of study population (n=100) was found to be 37.75±2.337weeks.
The mean body weight of study population was found to be 2466.40±579.437 grams.
Out of 100 subjects, 61(61%) were males and 39(39%) were females. Detailed demographic data are represented in [Table/Fig-2].
Demographic characteristics of the study population.
| S.No. | Characteristics | Neonates | NS n=41 | HS n=59 |
|---|
| 1 | Gender | Male | 61 | 27 (44.3%) | 34 (55.7%) |
| Female | 39 | 14 (35.9%) | 25 (64.1%) |
| 2 | Gestational Age (weeks) | Term | 61 | 29 (47.5%) | 32 (52.5%) |
| Preterm | 39 | 12 (30.8%) | 27 (69.2%) |
| Mean±SD | 38.15±1.918 | 37.47±2.569 |
| 3 | Body weight(grams) | 1500-2000g | 24 | 7 (29.2%) | 17 (70.8%) |
| >2000g | 76 | 34 (44.7%) | 42 (55.3%) |
| Mean±SD | 2539.76±560.238 | 2415.42±591.760 |
Commonly Administered Medications through PIV Catheter
Major drugs administered through PIVL during the study were Amikacin, Ampicillin, Piperacillin-Tazobactam, Phenytoin, Cefuroxime, Phenobarbitone, Cefotaxime etc.
Assessment of Effectiveness of Flush Solutions
Poisson regression was used to assess the effectiveness of flush solutions and the mean number of catheters replaced due to nonelective reasons in Normal Saline (NS) group was 1.12 times higher compared to Heparinized saline (HS) group, which was statistically not significant {95% Wald Confidence Interval: 0.771-1.634; p-value: 0.584}.
Other Factors Affecting Patency
Assessment of effect of gestational age on patency of catheter: The mean number of catheters replaced due to non-elective reasons in the preterm group is 1.004 times higher compared to term group, which was statistically not significant {95% Wald Confidence Interval: 0.677-1.489; p-value =0.983}.
Assessment of effect of body weight of neonates on patency of catheter: The mean number of catheters replaced due to non-elective reasons in 1500-2000g group is 1.124 times higher compared to >2000g group, which was statistically not significant {95% Wald Confidence Interval:0.715-1.769; p-value =0.612}.
Assessment of effect of site on patency of catheter: Seventy-seven catheters in hand and 23 catheters in the leg for the first catheter were studied for assessment of the effect of site on patency of catheter.
The mean number of catheters replaced due to nonelective reasons in hand group is 1.104 times higher compared to leg group, which was statistically not significant {95% Wald Confidence Interval: 0.679-1.792; p-value =0.612}.
Assessment of Safety
Safety was analysed by assessing the reason for removal of the catheters and occurrence of thrombocytopenia in the groups.
Assessment of reason for removal of catheters: Catheter removals in 47 neonates (79.6%) of HS group (n=59) and in 33 neonates (80.5%) of NS group (n=41) were due to non-elective reasons like phlebitis, obstruction/occlusion of the cannula or fluid leakage. Whereas catheter removals in 12 neonates (20.4%) of the HS group (n=59) and in 8 neonates (19.5 %) of the NS group (n=41) were due to elective reasons like removals due to discharge, starting IV fluids, end of therapy or dislocation.
Assessment of thrombocytopenia: The other parameter studied to assess the safety of flushing solutions was the occurrence of thrombocytopenia in the two groups. The cases of thrombocytopenia were more in HS group, i.e., 22 (37.3%) cases than in NS group 9 (22%). Cases of thrombocytopenia were observed significantly more in neonates with sepsis compared with no sepsis cases in both the groups. Details are represented in [Table/Fig-3]. In this study, the presence of sepsis was found to have an association with the occurrence of thrombocytopenia (Pearson Chi-square value–35.68; p-value <0.001). Stratified analysis was done to account for the presence of sepsis in the study groups and the frequency of thrombocytopenia was compared again. Results again confirmed that for each level of sepsis there was no significant difference between the frequencies.
Assessment of frequency of thrombocytopenia.
| Sepsis | | Thrombocytopeniapresent | Thrombocytopeniaabsent | Total |
|---|
| No sepsis | NS | 1 | 23 | 24 |
| HS | 1 | 26 | 27 |
| Total | 2 | 49 | 51 |
| Culture positive sepsis | NS | 5 | 6 | 11 |
| HS | 14 | 7 | 21 |
| Total | 19 | 13 | 32 |
| Culture negative sepsis | NS | 3 | 3 | 6 |
| HS | 7 | 4 | 11 |
| Total | 10 | 7 | 17 |
| Total | NS | 9 (22%) | 32 (78%) | 41 |
| HS | 22 (37.3%) | 37 (62.7%) | 59 |
| Total | 31 | 69 | 100 |
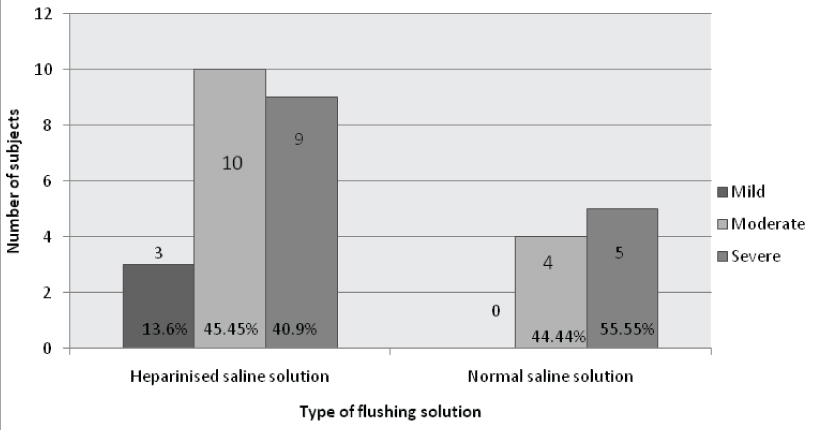
Details of the severity of thrombocytopenia observed in the study groups are described in [Table/Fig-4].
Assessment of severity of thrombocytopenia in NS and HS groups.
Discussion
We conducted a double blinded randomized trial to assess effectiveness and safety of HS versus NS in maintaining patency of PIVL in neonates at the NICU of a tertiary care hospital. Total of 100 neonates (HS group: 59; NS group: 41) were included in the study. The mean gestational age of neonates was 37.75±2.337 weeks and mean weight was 2466.40±579.437 grams. The mean number of catheters replaced due to non-elective reasons in NS group was found to be 1.12 times higher compared to HS group {p-value=0.584} thus, we found that HS and NS are equally efficacious. It may be the positive pressure of flushing solutions inside the catheter that prevents the occlusion or clotting of blood rather than anticoagulant properties of heparin at this concentration of 1U/ml. Our results are similar to that of the studies by Kotter, Paisley MK, Heilskov et al., Hanrahan et al., Mok et al., Arnts IJJ et al., and Brown K which also showed that there is no significant difference between both the groups [24-28,33,34]. In contrast to our study, Danek GD, Mudge et al., and Schultz et al., showed that there is a significant difference between both the groups and HS solution has longer patency compared to NS [23,29,35]. However, methods followed for analysis, baseline characteristics of the population and concentrations used for flushing varied among studies.
We assessed factors affecting patency of the catheters such as gestational age, body weight, and site of insertion, with respective flushing solutions, and found that these factors did not significantly affect the patency. These results are similar to that of the study by Arnts IJJ et al., [33]. A study by Brown K [34] showed the risk of catheter occlusion was inversely correlated with gestational age. This increase in occlusion in the lower gestational age infants (especially ≤ 30 weeks) may be due to smaller veins resulting in more susceptibility to occlusion.
A study in children suggested that the type of fluid and medications administered does not affect catheter lifespan [10], but this is not well studied in neonates. Brown K demonstrated an increased catheter occlusion with cefotaxime and vancomycin (compared with ampicillin and gentamycin) [34] and another report indicated gentamycin use to increase infusion site failure in neonates [1]. Major drugs administered through PIVL during our study were amikacin, ampicillin, piperacillin-tazobactam, phenytoin, cefuroxime, phenobarbitone, cefotaxime, cefoperazone, metronidazole, ciprofloxacin, ceftriaxone-sulbactam, fluconazole, gentamycin and cefazolin. However, the effect of patency with respect to each drug could not be studied in our study, since the majority of neonates were on multiple drugs.
Assessment of safety was analysed by assessing the reason for removal of the catheters and occurrence of thrombocytopenia in the groups 79.6% catheter removals in HS group (n=59) and 80.5% catheter removals in NS group (n=41) were due to non-elective reasons like phlebitis (inflammation of the vein with pain), obstruction/occlusion of the cannula (difficult or impossible to flush, inability to administer fluid in 3 seconds) or fluid leakage (around the cannula-insertion). Whereas, 12 (20.4%) catheter removals in HS group (n=59) and 8 (19.5%) catheter removals in the NS group (n=41) were due to elective reasons like removals due to discharge, starting IV fluids, end of therapy or dislocation. These results were similar to the study conducted by Arnts IJJ et al., [33].
To assess the safety of flushing solutions the other parameter studied was the occurrence of thrombocytopenia. The cases of thrombocytopenia were more in HS group that is 22 (37.3%) cases compared to 9 in NS group (22%). Cases of thrombocytopenia were observed significantly more in neonates with sepsis compare with no sepsis cases in both the flushing groups (Pearson Chi-square value –35.68; p-value<0.001). Out of the 22 cases of thrombocytopenia observed in HS group, sepsis was absent in one while 14 had Culture positive sepsis and 7 had Culture negative sepsis. Alternatively, out of the 9 cases of thrombocytopenia observed in the Normal saline group, sepsis was absent in one while 5 had Culture positive sepsis and 3 had Culture negative sepsis. Therefore, the contribution of flushing solutions to thrombocytopenia may be very minimal, since sepsis was found to play a major role. Results of the stratified analysis done to account for the presence of sepsis in the study groups again confirmed that for each level of sepsis there was no significant difference between the frequencies of thrombocytopenia.
This result is similar to other three studies which found no cases of heparin-induced thrombocytopenia or antibody formation associated with the use of low-dose heparin in neonates to maintain catheter patency [32,36,37].
Limitation
The required sample size could not be attained for both groups due to unavailability of complete data and limited time frame. The complications associated with IVL like phlebitis, thrombophlebitis, occlusion, infiltration, oedema, bleeding could not be studied in detail. Heparin induced thrombocytopenia could not be assessed since the 4-T score and HIT related lab tests were not performed. In addition, confounding factors like sepsis, drugs, and patient related factors were also present. Patency of Flushing solutions with respect to frequency could not be assessed because of non-uniformity in frequency of flushing. Results cannot be generalized in the neonates for the following reasons: a) Study did not include preterm neonates below 34 weeks; b) Size of the catheter studied was 24 G only; c) Low birth weight neonates <1500g were not included in the study; d) Only a single concentration of heparin solution (1 unit/ml) was studied.
Conclusion
The present study was conducted to assess the efficacy and safety of 1U/ml HS and 0.9% NS flushes in maintaining patency of 24 Gauze PIVL in neonates. The study showed that there is no significant difference in duration of patency between the solutions and no significant relationship with gestational age, body weight and site of insertion on patency of catheters. The frequency of thrombocytopenia was observed more in HS group than with NS group and it was not statistically significant. There were more mild, moderate and severe cases of thrombocytopenia in HS group. The occurrence of thrombocytopenia was attributed to sepsis in the study group.
Therefore; in the absence of a clear advantage of HS flush, PIVLs may be flushed using NS, as heparin may acquire a greater economical cost as costs involved in acquisition, preparation of HS solution and management of heparin associated complications and drug interactions are greater, which can be avoided to certain extent using Normal saline.