Liver haemangiomas are described to be the most frequent benign liver tumours, and are mostly incidental detections in asymptomatic individuals. Giant lesions can develop symptoms, and are associated with complications. Female sex hormones, particularly estrogen, are believed to influence haemangioma development and progression. The optimal management of giant haemangiomas during gravidity is inadequately illustrated in literature so far. Diagnosis of a symptomatic giant haemangioma during pregnancy presents a quandary for the clinicians; besides, the safety of conservative management during pregnancy has not been comprehensively investigated. Consensus on the indications for surgical resection of asymptomatic lesions is also lacking at present. Thus, we present a case of a giant hepatic haemangioma diagnosed during pregnancy, where in timely detection and close observation, could successfully diffuse an otherwise a ticking bomb to explode; and also review the literature, with an aim of alleviating the bewilderment on management of haemangioma in pregnancy that might exist in the mind of budding gynecologists.
Case Report
A 22-year-old primigravida, a known case of essential hypertension was admitted from the emergency of our hospital at 26 weeks of gestation, with complaints of discomfort and sense of fullness in upper abdomen and IUGR. She was diagnosed as having high BP records since 18 weeks of gestation. Her past and family history was unremarkable.
On examination, nothing was noteworthy in general examinations. However, on per abdomen examination, uterus was corresponding to 26 weeks of size, with a single live fetus in longitudinal lie with cephalic presentation. Also, tender hepatomegaly was present ~6 cm below right costal margin. No evidence of ascites was present. Urgent necessary referrals (medicine, hepatologists and surgeons) were taken. An ultrasound whole abdomen was performed followed by a confirmatory MRI, which showed a well-demarcated, homogenous lesion, hypo-intense lesion on T1-weighted images and hyper-intense on T2-weighted images with characteristic the “cotton-wool” aspect~ 11 x 10 x 9.5cm; these findings were evocative of a presence of hepatic Haemangioma [Table/Fig-1]. Patient and her relatives were adequately counselled in collaboration with the surgeons and the hepatologists about the prognosis of the entity with respect to her pregnancy and vice versa. Also, the treatment options available were briefed upon and the choice of management approach made for her including close observation throughout pregnancy and elective resection postpartum, was informed. This decision was made after weighing the concern of rupture against the risks of surgery to both mother and the fetus.
MRI report of the patient confirming the diagnosis of hepatic haemangioma.
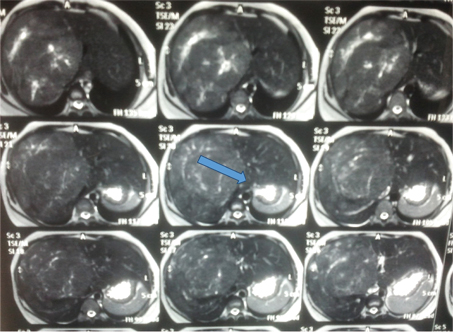
She was being closely monitored for maternal and fetal well-being henceforth. Her BP kept in the range of 152/102 to 160/108; hence tablet labetalol in a dose of 200mg was started thrice a day. Her 24 hour urinary protein level was less than 500mg in 24 hours. Fundus examination showed normal glow, clear media and a normal optic disc configuration with normal vascularity. Her biochemical parameters (haemogram, liver function test, kidney function test, blood glucose levels, and platelet count and coagulation profile) remained within normal limits [Table/Fig-2]. The obstetric USG confirmed asymmetrical IUGR, though Doppler velocimetry was normal.
Serial investigations of the patient in hospital.
| Parameters | 26 weeks | 30 weeks | 32 weeks | 34 weeks |
|---|
| Haemoglobin(g/dl) | 10.3 | 10.2 | 10.4 | 10 |
| TLCa(per microliter (mcL) | 7600 | 8200 | 7500 | 8000 |
| Platelet count (per microliter) | 1,00,000 | 86000 | 74,000 | 62,000 |
| Blood urea (mg/dL) | 26 | 27 | 26 | 21 |
| Serum creatinine (mg/dL) | 0.7 | 0.7 | 0.6 | 0.8 |
| Uric acid (mg/dL) | 1.4 | 1 | 1.6 | 0.8 |
| LFTbTotal bilirubin (mg/dl)/ SGOTc(units/L)/ SGPTd(units/L) / SALPe(IU/L) | 0.2/42/38/146 | 0.3/72/78/200 | 0.5/118/106/279 | 0.6/140/123/349 |
| SGOTc(units/L)/ SGPTd(units/L) / SALPe(IU/L) | | | | |
| 24 hour urine protein (mg/day) | 250 | 266 | 254 | 257 |
| LDHf | 298 | 312 | 304 | 300 |
| Coagulation profile | WNLg | WNLg | WNLg | WNLg |
| USGh | SLFi,BPDj-23+4wks, ACk-22+4wks,FLl-22+5wks, liquor adequate,noGCAm,dopplernormal | SLF,BPD-25+5wks, AC-24+6,FL-25wks,liquoradequate | SLF,BPD-27+2wks,AC-26+4wks,FL-27wks, liquor adequate, | SLF,BPD-29wks,AC-28+4,FL-28+4, liquor adequate, doppler normal |
a) Total leucocyte count b) Liver function tests c) Serum glutamic oxaloacetic transaminase d) Serum glutamate pyruvate transaminase e) Serum Alkaline phosphatase f) Lactate Dehydrogenase g) Within normal limits h) Ultrasonography i) Single live fetus j) Biparietal diameter k) Abdominal circumference l) Femur Length m) Gross congenital anomaly
All investigations were repeated at weekly intervals to monitor progression of disease and to rule out HELLP syndrome, as she often complained of pain in epigastric region (an ominous sign in a pregnant patient with high BP records). Biophysical testing was started at 30th week in view of fetal IUGR. She was advised to keep a strict daily kick count.
Platelet counts were slowly and gradually decreasing over a period of time from 26 weeks to 34 weeks, the cause of which could not be delineated, as some forms of hepatic haemangiomas are also known to develop thrombocytopenia due to an inherent pathology in the vascular mass itself (Kasabach-Merritt syndrome). An elective cesarean delivery was thus performed bearing in mind the worsening epigastric pain, decreasing platelet count and fetal IUGR, so as to avoid possible rupture of the haemangioma during the second stage of labor (had she been allowed a vaginal delivery).
She delivered a live baby boy of 1.4 kg weight with good efforts and cry. It was admitted in NICU being very low birth weight baby for observation. Also during the surgery, a large mass ~11*10*10 cm, suggestive of haemangioma was seen arising from the liver. The blood loss during LSCS was average. She had an uneventful postoperative period. Both mother and baby were discharged after a week in a satisfactory general condition.
The patient was advised to follow up in case of acute abdomen or related symptoms as was planned in consultation with the interventional radiologist and the surgeons. Repeat MRI after three months showed the mass further decreasing to attain dimensions of 10 x 8 x 9 cm.
The patient is being diligently followed up by us; recently Cu-T 380 A was inserted on her request after 11 months of operative delivery. Till now she has developed no new complaint, her baby is doing fine; also the pain that she felt in the epigastric region during pregnancy has subsided. Follow up MRI was also done after 10 months of delivery depicting further regression of Haemangioma (size 8 x 9 x 7cm). She is regularly seeking surgery consultation, as well as interventional radiological advice for definitive management of haemangioma and is recuperating well till date.
Discussion
Haemangioma is the commonest benign mesenchymal tumour of the liver consisting of clusters of blood-filled cavities, lined by endothelial cells, nurtured by the hepatic artery. These congenital vascular malformations stem from eclectic growth of the blood vessels that are atypical or irregular in arrangement and size [1,2]. They can be either capillary or cavernous haemangiomas. When these lesions enlarge to a momentous size, more than 5 cm, they are referred to as giant haemangiomas. The reported incidence is 0.4%-20% of the total population [2,3]. These are more common in the right lobe of the liver than in the left lobe, as was in the present case. Mainly, they are mono-lesions, but multiple-lesions are possible [4-9]. Women are more vulnerable to develop hepatic haemangiomas (4.5:1 to 5:1 ratio of female to male cases) in fourth to fifth decades of life [5,7].
The precise cause of hepatic haemangiomas is not known, it may be congenitally determined, implying a possible genetic association. An abnormal vasculogenesis and angiogenesis may be involved in the pathogenesis, incited by an upsurge in angiogenic factors such as Vascular Endothelial Growth Factors (VEGF) and Matrix Metalloproteinases (MMPs), and a diminution in anti-angiogenic factors. Tumour growth is fostered by high blood estrogen level during puberty, pregnancy, oral contraceptive use, and androgen treatment. Especially, an increase in size of more than 0.5cm is considered significant [1,2,4,7].
The vast majority are asymptomatic, often being detected accidentally during imaging investigations for various disparate pathologies [1-3].
Symptoms are felt by up to 90% of women with giant haemangiomas in pregnancy [10-12]. Though rapid growth, haemorrhagic, degenerative, inflammatory and mechanical complications have been reported during pregnancy, but most of these tumours can be safely observed during pregnancy [10-13]. Largely symptomatology includes right upper quadrant pain/discomfort or fullness, decreased appetite, premature satiation sensation, nausea, vomiting, postprandial bloating. These are often with the physiological symptoms of pregnancy.
Rarely, large tumours rupture spontaneously or after blunt trauma when patients may present with signs of circulatory shock and haemoperitoneum. In our case presence of a big haemangioma with pre-eclampsia was also a vulnerable situation, as it might increase in size under the effect of estrogens and also may rupture spontaneously. Spontaneous rupture is the most dreaded complication owing to a mortality rate approaching 80% [14-17]. Accelerated growth, increased intra-abdominal pressure and direct contact with the gravid uterus are all plausible mechanisms for spontaneous rupture or worsening symptoms during antenatal period. Increased intraabdominal pressure might lead to an intrapartum rupture of such giant haemangiomas as well. Our patient’s tumour was assessed as being high risk for complications during pregnancy due to its size, exophytic nature and subumbilical position. Therefore, she was admitted and kept under close observation
Also, giant hepatic haemangiomas are associated with thrombocytopenia and intravascular coagulation leading to Kasabach-Merritt syndrome [2-5]. Thrombocytopenia can result from sequestration and destruction of platelets in large lesions. Hypo-fibrinogenemia may be a consequence of intratumoural fibrinolysis. Patients developing a new onset of abdominal pain in pregnancy who were relatively asymptomatic before, like the described case, must undergo imaging studies to rule out or confirm any increase in size of the lesion.
Imaging studies used in the diagnosis of hepatic haemangiomas during pregnancy include ultrasonography, and magnetic resonance imaging [2-6,15-18]. On conventional ultrasound, the lesion appears as a hyperechoic homogenous nodule, with well-defined margins and posterior acoustic enhancement, like in the present case. These findings were confirmed by MRI where in a well-demarcated, homogenous lesion, hypointense lesion on T1-weighted images and hyperintense on T2-weighted images, the “cotton-wool” aspect was noted ~11x10x9.5 cm. Increasing the echo time (TE) led to a rise in this signal, differentiating it from malignant lesion. Managing hepatic haemangiomas often present a tricky situation in front of the clinicians, as the risk and need of surgery have to be carefully balanced against the complications and feto-maternal outcome [1,2,5-8,14-18]. In most asymptomatic cases, they should be followed up by means of periodic radiological examination. Surgery is often restricted to specific situations like spontaneous or traumatic rupture with haemoperitoneum, intratumoural bleeding and consumptive coagulopathy (Kasabach-Merritt syndrome). However, with knowledge and high clinical suspicion in a women presenting with acute abdominal pain, spontaneous rupture of a hepatic haemangioma should be considered and the needful be done.
Other advances in management include arterial embolization, surgical ligation of feeding vessels, radiofrequency ablation [1-5]. Hepatic irradiation with a dose of 15-30 Gy in 15-22 fractions over several weeks has been used to treat symptomatic haemangiomas. Tumour regression and symptom relief were noted in most patients, with minimal morbidity [1-6]. But the safety and efficacy of these procedures during pregnancy have been a concern for many. In our patient conservative approach was followed after being adequately counseled of definitive treatment options. This case is being reported for its rare presentation combined with therapeutic dilemma. There is little available literature describing the outcome of liver haemangioma resection during pregnancy. A larger observational cohort study in non-pregnant individuals concluded that haemangioma resection was justified (owing to minimal morbidity and no mortality) in the 13 operated patients. However, these authors also detected that no tumours < 5 cm increased in size or ruptured in patients who received conservative management [1]. Obviously, extrapolation of these conclusions to our patient would be erroneous, as the large peripheral exophytic tumour was >5 cm and she was pregnant. Also, [Table/Fig-3] illustrates the reported cases of successful management of hepatic haemangiomas in pregnancy with conservative approach, exemplifying the importance of meticulous planning, close monitoring and watchful expectancy in such patients in averting disastrous events to have a positive feto-maternal outcome [12,13,19].
Case reports showing conservative management in hepatic haemangioma in pregnancy in last decade.
| Authors(Year) | Age/Parity | Clinicalprofile | Size and location of haemangioma | Complications during pregnancy | Management | Delivery | Maternaloutcome | Fetal outcome |
|---|
| Au WY and Liu CL [12] | 33y/G2P1L1 | Incidental finding on USG at 16 weeks | 3cm/rightlobe | None | observation | El LSCS due totriplet gestation | good | good |
| Cobey FC and Salem RR [13] | 39yr/G5P4L4 | Incidental finding on USG at 17 weeks | 10cm/NS | None | Observationf/b enucleation 1yr post- partum | LSCS | good | good |
| Cobey FC and Salem RR [13] | 31y/G5P4L4 | Rt upper quadrant pain at 36 weeks | 3cm/NS | None | observation | LSCS | good | good |
| Cobey FC and Salem RR [13] | 36Y/G3P2L2 | Incidental finding on USG priorto pregnancy | 8cm/NS | None | observation | Vaginal | good | good |
| Spitzer D et al., [19] | 31y/G1 | Incidental findingon USG prior to pregnancy | 14cm/right lobe | None | observation | ElectiveLSCS(to avoid possible rupture) | good | good |
| Present case | 22y/G2A1 | Incidental findingon USG at 26 weeks pregancy | 11cm/right lobe | Preeclampsia,IUGR and thrombocymiddleenia | observation | LSCS | good | 1.4 kg live boy baby –NICU being VLBW |
Conclusion
With the advent of refined methods of diagnosis to identify their incidence and prevalence in the general population, hepatic haemangiomas that would have gone undetected in the past, are now being diagnosed. Also, modern techniques in liver surgery could make the surgical treatment of such cases safer than in past, with relatively low peri-operative morbidity. However, the low rates of life-threatening events associated with conservative management of haemangiomas challenges the validity of surgical resections in pregnancy owing to a high risk of haemorrhage and maternal and fetal demise.
a) Total leucocyte count b) Liver function tests c) Serum glutamic oxaloacetic transaminase d) Serum glutamate pyruvate transaminase e) Serum Alkaline phosphatase f) Lactate Dehydrogenase g) Within normal limits h) Ultrasonography i) Single live fetus j) Biparietal diameter k) Abdominal circumference l) Femur Length m) Gross congenital anomaly
[1]. Hann A, Osenda E, Reade JA, Economides D, Sharma D, Case report: successful open resection of a symptomatic giant liver haemangioma during the second trimester of pregnancyJournal of Surgical Case Reports 2016 11:1-3. [Google Scholar]
[2]. Miura JT, Amini A, Schmocker R, Nichols S, Sukato D, Winslow ER, Surgical management of hepatic haemangiomas: a multi-institutional experienceHPB (Oxford) 2014 16(10):924-28. [Google Scholar]
[3]. Bajenaru N, Balaban V, Săvulescu F, Campeanu I, Patrascu T, Hepatic haemangiomaJournal of Medicine and Life 2015 8(Spec Issue):4-11. [Google Scholar]
[4]. Salati SA, Kadi AA, Hepatic haemangioma –a case report and review of literatureEast and Central African Journal of Surgery 2015 20(2):110-22. [Google Scholar]
[5]. Kim JM, Chung WJ, Jang BK, Hwang JS, Kim YH, Kwon JH, Haemorrhagic haemangioma in the liver: A case reportWorld J Gastroenterol 2015 21(23):7326-30. [Google Scholar]
[6]. Toro A, Mahfuz AE, Ardiri A, Malaguernera M, Malguernera G, Loria F, What is changing in indications and treatment of haemangiomas- a review?Annals of Hepatology 2014 13(4):327-39. [Google Scholar]
[7]. Kneuertz PJ, Marsh JW, de Jong MC, Covert K, Hyder O, Hirose K, Improvements in quality of life after surgery for benign hepatic tumors: results from a dual center analysisSurgery 2012 152(2):193-201. [Google Scholar]
[8]. Ebina Y, Hazama R, Nishimoto M, Tanimura K, Miyahara Y, Morizane M, Resection of giant liver haemangioma in a pregnant woman with coagulopathy: Case report and literature reviewJournal of Prenatal Medicine 2011 5(4):93-96. [Google Scholar]
[9]. Glinkova V, Shevah O, Boaz M, Levine A, Shirin H, Hepatic haemangiomas: possible association with female sex hormonesGut 2004 53(9):1352-55. [Google Scholar]
[10]. Holst B, McGuinness E, Morris-Stiff G, Spontaneous peripartum liver haemorrhage presenting as fetal distressGrand Rounds 2013 13:42-46. [Google Scholar]
[11]. Ribeiro MAF, Papaiordanou F, Gonçalves JM, Chaib E, Spontaneous rupture of hepatic haemangiomas: A review of the literatureWorld J Hepatol 2010 2(12):428-33. [Google Scholar]
[12]. Au WY, Liu CL, Growth of giant hepatic haemangioma after triplet pregnancyJournal of Hepatology 2005 42(5):781 [Google Scholar]
[13]. Cobey FC, Salem RR, A review of liver masses in pregnancy and a proposed algorithm for their diagnosis and managementAm J Surg 2004 187(2):181-91. [Google Scholar]
[14]. ACOG Committee on Obstetric PracticeACOG Committee Opinion No. 474: nonobstetric surgery during pregnancyObstet Gynecol 2011 117(2 Pt 1):420-21. [Google Scholar]
[15]. Saegusa T, Ito K, Oba N, Matsuda M, Kojima K, Tohyama K, Enlargement of multiple cavernous haemangioma of the liver in association with pregnancyIntern Med 1995 34(3):207-11. [Google Scholar]
[16]. Graham E, Cohen AW, Soulen M, Faye R, Symptomatic liver haemangioma with intra-tumour haemorrhage treated by angiography and embolization during pregnancyObstet Gynecol 1993 81(5 Pt 2):813-16. [Google Scholar]
[17]. Yamagata M, Kanematsu T, Matsumata T, Utsunomiya T, Ikeda Y, Sugimachi K, Management of haemangioma of the liver: comparison of results between surgery and observationBr J Surg 1991 78(10):1223-25. [Google Scholar]
[18]. Mocchegiani F, Vincenzi P, Coletta M, Agostini A, Marzioni M, Baroni GS, Prevalence and clinical outcome of hepatic haemangioma with specific reference to the risk of rupture: a large retrospective cross-sectional studyDig Liver Dis 2016 48(3):309-14. [Google Scholar]
[19]. Spitzer D, Krainz R, Graf AH, Menzel C, Staudach A, Pregnancy after ovarian stimulation and intrauterine insemination in a woman with cavernous macrohaemangioma in liver: a case reportJ Reproductive Medicine 1997 42(12):809-12. [Google Scholar]