Medicopsis romeroi (M. romeroi) (syn. Pyrenochaeta romeroi) is a rare melanized fungus causing subcutaneous/localized invasive phaeohyphomycosis in immunocompromised patients. We present a rare case of subcutaneous phaeohypho-mycotic cyst caused by M.romeroi in a diabetic male with lepromatous leprosy. Molecular identification was performed by sequencing of Internal Transcribed Spacer (ITS) region and D1/D2 region of Large Ribosomal Subunit (LSU).
Dapsone, Diabetes, Mycosis
Case Report
A 48-year-old diabetic male patient, resident of Bihar (India), daily wage construction labour by occupation was diagnosed with lepromatous leprosy in March 2016. He was on anti-leprosy treatment regimen of dapsone, rifampicin and glypazide. He was admitted in October 2016 to a tertiary care hospital in Delhi, with chief complaints of pain in knee joint, red scaly raised lesions all over the body since last one month. On admission, he was clinically diagnosed with Erythema Nodosum Leprosum (ENL). The patient also complained of a painless progressively growing nodule on the lateral aspect of the left foot since last three months. There was history of frequent contact with debris of construction material.
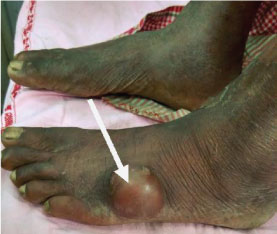
On examination, red macular lesions were observed all over the body. The leprosy status was reconfirmed by Slit Skin Smear (SSS) examination. Patient consent was taken for further diagnosis of the fluctuating cyst. The fluctuating cystic swelling was about 2×2 inches in diameter with ill defined margins [Table/Fig-1]. Routine investigations revealed Haemoglobin - 6.6 mg/dl, leukocyte count 12,200 cells/cumm, differential count; neutrophils 68%, lymphocytes 25%, monocytes 4%, eosinophils 3%, basophil 0%, platelets 2.1 L/cumm and Erythrocyte Sedimentation Rate (ESR) 48 mm/hour, fasting blood sugar 320 mg/dl, blood urea 21 mg/dL, serum creatinin 0.40 U/L, Serum Glutamic Pyruvic Transaminase (SGPT) 10 U/L, ALP 71 U/L and serum electrolyte (Na+ 139 mmol/L and K+ 4.4 mmol/L). The tests were negative for HIV and Hepatitis B.
Showing fluctuating cystic swelling over left foot.
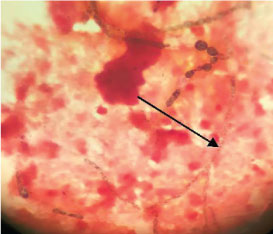
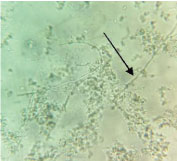
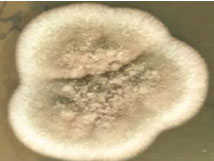
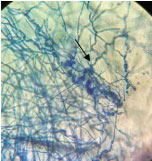
On the day of admission (day1) the cyst aspirate was sent for microbiological examination to the Microbiology laboratory for Gram’s stain, culture and sensitivity. Microscopic examination of Gram stain smear showed inflammatory cells and a few septate fungal hyphae in a background of necrotic material [Table/Fig-2]. The pus sample was also stained by Ziehl Neelsen stain and acid fast bacilli with characteristic cigar bundle arrangement were observed. A 20% KOH preparation of the sample revealed fungal elements [Table/Fig-3]. The sample was inoculated on 5% Sheep Blood Agar (SBA), Macconkey agar and incubated at 37°C.2 set of tubes of Sabouraud’s Dextrose Agar (SDA) were inoculated and 2 were incubated at 25°C and 2 were incubated at 37°C. A repeat sample was obtained on day 2 for confirmation of the above findings. Finally on day 4, incision and drainage of the cyst was carried out. All the above tests were repeated. Bacterial culture was sterile. A white, velvety growth was observed on SDA (at 25°C and 37°C) after eight days of incubation of the first sample. The colour gradually turned brown after two weeks [Table/Fig-4]. Lactophenol Cotton Blue (LPCB) mount revealed brown/black colour, tortuous hyphae. No conidia were observed on hyphae on Potato Dextrose Agar (PDA) [Table/Fig-5]. Identical microscopic findings and growth on culture were observed from all the samples. Therefore, the culture was subjected to molecular identification.
Gram’s stain showing black coloured fungal hyphae (100X).
A 20% KOH showing growth of filamentous fungal element (40X).
Phaeoid fungus after eight days of incubation on SDA media.
Showing tortuous fungal hyphae under LPCB stain (40X).
Treatment should be started after molecular identification. Surgical incision and drainage of the lesion was carried out and antifungal therapy with itaconazole was started. The patient Left Against Medical Advice (LAMA) from the hospital on the 15th day of hospital admission day and the follow up of the patient was not continued.
Molecular Identification
Molecular identification was performed by sequencing of Internal Transcribed Spacer (ITS) region and D1/D2 region of Large Ribosomal Subunit (LSU). DNA was extracted from seven days old culture using tris-saturated phenol, chloroform and isoamyl alcohol (25:24:1) and ethanol precipitation. The DNA pellet was dried and suspended in 75 μl of sterile nuclease-free water and treated with 06 μl (10 mg/ml) of RNAase (Sigma-Aldrich, Co., St. Louis, USA) for 1 hour at 37°C. The purified DNA of the pathogenic organism was subjected to PCR amplification of ITS region using pan fungal primers ITS1 (5’-TCC GTA GGT GAA CCT GCG G-3’) and ITS4 (5’-TCC TCC GCT TAT TGA TAT GC-3’) and for D1/D2 region using primers NL1 (5’-GCA TAT CAA TAA GCG GAG GAA AAG-3’), NL4 (5’-GGT CCG TGT TTC AAG ACG G-3’) [1,2].
The amplified fragments were purified (Wizard SV Gel and PCR Clean-up System, Promega) and sequenced using the cycle sequencing kit (BigDye Terminator v3.1 cycle sequencing kit RR100; Applied Biosystems, Foster City, CA, USA) and the final product was sequenced on an ABI 3130xL Genetic analyser (Applied Biosystems). DNA sequences were analysed with Sequencing Analysis 5.3.1 software (Applied Biosystems, Foster City, CA, USA) and the aligned sequences were subjected to GenBank Basic Local Alignment Search Tool (BLAST) searches (http://www.ncbi.nlm.nih.gov/BLAST/Blast.cgi) for species identification. BLAST searches confirmed the isolate as M. romeroi with 100% identity of both ITS and D1/D2 sequences to M. romeroi strains. ITS sequences of our isolate GenBank accession no. KU361135 matched with 100% identity with three strains (CNRMA9.134, Kw596 and IP 888.65) of M. romeroi (GenBank accession no. KP132405.1, DQ836803.1 and FN826905.2). However, the D1/D2 sequence of our isolate with Gene Bank accession no. KU361119 matched with 99%-100% identity with four stains (CBS 128765, CBS 123975, CBS 132878 and L2572) of M. romeroi (Gene Bank accession no. KF015621.1, KF015623.1, KF015622.1 and KC288120.1).
Discussion
Opportunistic subcutaneous fungal infections are increasing in present time. Some of the highly recalcitrant, disseminated infections appeared to be associated with immunosuppression, namely Diabetes Mellitus (DM), AIDS, organ transplantation recipients, anticancer therapy etc., [3]. Phaeohyphomycosis is a heterogeneous group of superficial or deep fungus with black filaments that often causes opportunistic infections [4–6]. The members of the genus Medicopsis romeori (syn. Pyrenochaeta romeroi) are widely distributed in soil and plant debris [7]. The fungus is generally inoculated through direct trauma and remains localized to cutaneous and sub-cutaneous tissue. M.romeroi is often isolated from mycetoma. Recently, it has also been found as a causative agent of phaeohyphomycotic cyst [8-15].
Leprosy is an immunocompromised condition leading to many superimposed opportunistic infections of bacterial and fungal origin [16-19]. Phaeohyphomycosis enrolls a heterogenous group of cutaneous, subcutaneous and systemic fungal infections caused by fungi with melanised hyphae or yeast cells [4,6,7,15]. Medicopsis is a genus of Coelomycetes having conidial fruit bodies covered with setae. Recently, the genus has been reclassified into other genera. The species are characterized by the presence of rapidly growing flat, velvety or floccose colonies producing dark olive-gray, aerial hyphae with olivaceous black reverse. Pyrenochaeta romeroi and Pyrenochaetamackinnonnii were accommodated in the new genera Medicopsis and Nigrograna, respectively, based on the analysis of ITS, D1/D2, β-tubulin, and chitin synthase 1 gene sequences. They are saprophytic fungus found in soil and plants that can be introduced into deeper tissues by trauma [15].
The number of identified pathogenic agents has steadily increased with the growing number of immunocompromised hosts [6,20-23]. There are many reports of fungal infection in immunocompromised patients [24-33]. [Table/Fig-6] shows review of M. romeroi infections in patients from India.
Review of Medicopsis romeroi infection in patients from India.
| Author, year, Place | Area involved | Underlying condition | Treatment given | Outcome |
|---|
| Kulkarni M et al., India [9] | Left thigh, left calf, and right shin | Kidney transplant recipient | Surgical excision, voriconazole | Survived |
| Badali H et al., India [11] | Right forearm | Verrucus plaque | Incision and drainage alone | Survived |
| Thammayya A et al., India [24] | Foot | NA | NA | NA |
| Yadav S et al., India [28] | Left foot | Diabetes | Incision and drainage, itraconazole | Survived |
| Babu K, India [31] | Left eye | Immuno-competent | Intra-vitreal voriconazole and amikaci | Survived |
NA= Not available.
Clinical lesions of deep phaeohyphomycosis are nonspecific, sometimes deceptive and usually present as subcutaneous indolent nodule. The literature shows that in absence of standard therapy, surgery is usually successful. Complete removal of the encapsulated structure can be curative in case of sub cutaeneous cyst. Although occasionally medical therapy alone is successful, combined medical-surgical therapeutic approaches are preferable, and medical treatment is reserved for patients with multiple abscesses and where surgery is contraindicated.
M. romeroi has been shown to be resistant to broad spectrum anti fungal such as amphotercin B, fluconazole, itraconazole and caspofungin [11,13]. The treatment should be tailored according to the location and size of the lesion.
Conclusion
It was concluded that combined surgical and antifungal treatment can prevent early deep-tissue involvement. The present case was treated with itraconazole and surgical debridement. Increased awareness among clinician and microbiologist/mycologist of Medicopsis romeroi infection will go a long way in rendering timely patient care.
NA= Not available.
[1]. White TJ, Bruns T, Lee S, Taylor J, Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds)PCR Protocols: A Guide to Methods and Applications 1990 San Diego, CAAcademic Press:315-22. [Google Scholar]
[2]. Kurtzman CP, Robnett CJ, Identification of clinically important ascomycetous yeasts based on nucleotide divergence in the 5’ end of the large-subunit (26S) ribosomal DNA geneJ Clin Microbiol 1997 35:1216-23. [Google Scholar]
[3]. Wang X, Wang W, Lin Z, Wang X, Li T, Yu J, CARD9 mutations linked to subcutaneous phaeohyphomycosis and TH17 cell deficienciesJ Allergy Clin Immunol 2014 133(3):905-08. [Google Scholar]
[4]. Ajello L. in: Proceedings of the Third International Conference on Mycosis. Scientific publication no.304, Pan American Health Organization Washington DC; 1975;126-133 [Google Scholar]
[5]. Rinaldi MG, PhaeohyphomycosisDermatol Clin 1996 14(1):147-53. [Google Scholar]
[6]. Borelli D, Opportunistic fungi producers of gray colonies and mycetomataDermatologica 1979 159:168-74. [Google Scholar]
[7]. Hoog GS, Guarro J, Gene J, Figueras MJ, Atlas of Clinical Fungi 2000 2nd ed [Google Scholar]
[8]. Abdolrasouli A, Ximena G, Anita J, Gordon JM, Nicholas F, Berge S, Subcutaneous phaeohyphomycosis cyst associated with Medicopsisromeroi in an immunocompromised hostMycopathologia 2016 181(9):717-721. [Google Scholar]
[9]. Kulkarni M, Jamale T, Hase N, Ubale M, Keskar V, Jagadish PK, Subcutaneous phaeohyphomycosis caused by Pyrenochaeta romeroi in a kidney transplant recipient: a case reportExp Clin Transplant 2016 15(2):226-27. [Google Scholar]
[10]. Chan YY, Tan AL, Tan BH, Subcutaneous abscess due to Pyrenochaeta romeroi in a renal transplant recipientSingapore Med J 2014 55(4):e64-66. [Google Scholar]
[11]. Badali H, Chander J, Gulati N, Attri A, Chopra R, Najafzadeh MJ, Subcutaneous phaeohyphomycotic cyst caused by Pyrenochaeta romeroiMed Mycol 2010 48(5):763-68. [Google Scholar]
[12]. Girard C, Dereure O, Rispail P, Durand L, Guilhou JJ, Subcutaneous phaeohyphomycosis due to Pyrenochaeta romeroi in a patient with leprosyActa Derma Venereol 2004 84:154-55. [Google Scholar]
[13]. Cerar D, Malallah YM, Howard SJ, Bowyer P, Denning DW, Isolation, identification and susceptibility of Pyrenochaeta romeroi in a case of eumycetoma of the foot in the UKInt J Antimicrob Agents 2009 34(6):617-8. [Google Scholar]
[14]. Khan Z, Ahmad S, Kapila K, Ramaswamy N, Alath P, Joseph L, Pyrenochaeta romeroi: a causative agent of phaeohyphomycotic cystMed Microbiol 2011 60:842-46. [Google Scholar]
[15]. Chowdhary A, Perfect J, Sybren GdH, Black molds and melanized yeasts pathogenic to humansCold Spring Harb Perspect Med 2014 5(8):a019570 [Google Scholar]
[16]. Basílio FM, Hammerschmidt M, Mukai MM, Werner B, Pinheiro RL, Moritz S, Mucormycosis and chromoblastomycosis occurring in a patient with leprosy type 2 reaction under prolonged corticosteroid and thalidomide therapyAn Bras Dermatol 2012 87(5):767-71. [Google Scholar]
[17]. Yang S, Makredes M, O’Donnell P, Levin NA, A case of Hansen disease presenting as tinea versicolourDermatology Online Journal 2013 19(4):7 [Google Scholar]
[18]. Segundo JB, da Silva MA, Filho WE, Nascimento AC, Vidal FC, Bezerra GF, Cerebral aspergillosis in a patient with leprosy and diabetes: a case reportBMC Res Notes 2014 7:689 [Google Scholar]
[19]. Dashatwar D, Kar S, Gangane N, Pol V, Madke B, Kulkarni S, Chromoblastomycosis in a resident of a leprosariumLepr Rev 2015 86(1):102-07. [Google Scholar]
[20]. Andre M, Brumpt MV, Destombes P, Segretain G, Fungal mycetoma with black grains due to Pyrenochaeta romeroi in CambodiaBull Soc Pathol Exot Fil 1968 61(1):108-12. [Google Scholar]
[21]. Serrano JA, Pisano ID, Lopez FA, Black grain mini-mycetoma by Pyrenochaeta mackinnonii, the first clinical case of eumycetoma reported in Barinas state, Venezuela-Clinical-histological features and case treatmentJ Mycol Méd 1998 8:34-39. [Google Scholar]
[22]. Young NA, Kwon-Chung KJ, Freeman J, Subcutaneous abscess caused by Phoma sp. resembling Pyrenochaeta romeroi: unique fungal infection occurring in immunosuppressed recipient of renal allograftAm J Clin Pathol 1973 59(6):810-16. [Google Scholar]
[23]. Foulet F, Duvoux C, de Bievre C, Hezode C, Bretagne S, Cutaneous phaeohyphomycosis caused by Veronaea bothryosa in a liver transplant recipient successfully treated with itraconazoleClin Infect Dis 1999 29(3):689-90. [Google Scholar]
[24]. Thammayya A, Sanyal M, Basu N, Pyrenochaeta romeroi causing mycetoma pedis in IndiaJ Indian Med Assoc 1979 73(3-4):66-67. [Google Scholar]
[25]. Thiyagarajan UM, Bagul A, Nicholson ML, A nodulo-cystic eumycetoma caused by Pyrenochaeta romeroi in a renal transplant recipient: a case reportJ Med Case Rep 2011 5:460-61. [Google Scholar]
[26]. Maiti PK, Haldar PK, Mycetomas in two different trauma-prone parts of body: a study of 212 casesIndian J Med Microbiol 1998 16:19-22. [Google Scholar]
[27]. Clancy CJ, Wingard JR, Hong Nguyen M, Subcutaneous phaeohyphomycosis in transplant recipients: review of the literature and demonstration of in vitro synergy between antifungal agentsMed Mycol 2000 38:169-75. [Google Scholar]
[28]. Yadav S, Agarwal R, Singh S, Goel S, Pyrenochaeta romeroi causing subcutaneous phaeohyphomycotic cyst in a diabetic femaleMed Mycol Case Rep 2015 8:47-49. [Google Scholar]
[29]. Hasio Y-W, Chia J-H, Lu C-F, Chung W-H, Molecular diagnosis and therapeutic experience of subcutaneous Pyrenochaeta romeroi infection: a case report and review of the literatureInternational Journal of Dermatology 2013 52(10):1237-40. [Google Scholar]
[30]. Babu K, Murthy PR, Prakash PY, Kattige J, Rangaswamy S, Murthy VR, Chronic endophthalmitis due to Pyrenochaeta romeroi in an immunocompetent host-a case report from southern IndiaRetin Cases Brief Rep 2014 8(3):197-99. [Google Scholar]
[31]. Ocampo MA, Kanitakis J, Bienvenu AL, Chauvet C, Euvrard S, Phaeohyphomycosis caused by Pyrenochaeta romeroi mimicking a plantar wart in a kidney transplant recipientTranspl Infect Dis 2012 14(6):E173-74. [Google Scholar]
[32]. Thiyagarajan UM, Bagul A, Nicholson ML, A nodulo-cystic eumycetoma caused by Pyrenochaeta romeroi in a renal transplant recipient: A case reportJ Med Case Reports 2011 5:460 [Google Scholar]
[33]. Ferrer C, Pérez-Santonja JJ, Rodríguez AE, Colom MF, Gené J, Alio JL, New Pyrenochaeta species causing keratitisJ Clin Microbiol 2009 47(5):1596-98. [Google Scholar]