The main objective of vital pulp therapy is to eliminate inflammation of the coronal portion of the dental pulp and to preserve the vitality of the remaining radicular pulp [1,2]. Over the past many years, various pulpotomy medicaments like formocresol, ferric sulphate, glutaraldehyde, calcium hydroxide and Mineral Trioxide Aggregate (MTA) has been used in primary teeth which has its own advantages and disadvantages [3,4]. Buckley’s formocresol has long been considered the “gold standard” pulpotomy medicament. None of them have met the same degree of clinical effectiveness and success rates as compared to formocresol [5]. International Agency for Research on Cancer (IARC) classified formocresol as carcinogenic to humans [6]. The use of herbal medicine has been on rise for the past few years in dentistry for the treatment of many infectious diseases as it possess good antibacterial and anti-inflammatory properties [7]. Therefore, this systematic review aims to compare the clinical and radiographic success of herbal medicine versus standard pulpotomy medicaments in primary teeth.
Materials and Methods
Literature Search
A detailed search was undertaken to identify all randomized controlled clinical trials which evaluated the clinical and radiographic success or outcome of herbal medicine compared to standard pulpotomy medicaments in primary molars. The articles which were published for the past ten years were included in the search. Electronic Databases like PubMed, Cochrane library, LILACS, Google scholar, SIGLE and Science Direct were searched until May 2017 and hand search was done in International Journal of Paediatric Dentistry (September 2011-March 2016), Journal of Clinical Paediatric Dentistry (September 2011–March 2016) and International Journal of Current Research and Academic Review (September 2013-March 2016) and in ongoing Trials Registers like US National Institutes of Health Trials Register, the WHO Clinical Trials Registry Platform, Clinical Trial Registry of India. Reference list of the identified randomized trials were also checked for possible additional studies.
Search Strategy
Child or children, pulpotomy or dental pulp therapy or vital pulp therapy, deciduous teeth or primary teeth or primary molars, Herbal medicine or garlic or Allium sativum or ankaferd blood stopper or haemostatic agent or propolis, pulpotomy medicaments or formocresol or calcium hydroxide or MTA or Biodentine.
PICO Analysis
Population: Children undergoing pulpotomy in primary teeth or deciduous teeth.
Intervention: Herbal medicine
Comparison: Standard pulpotomy medicaments
Outcomes: Clinical success/radiographic success
Inclusion Criteria
All clinical trials and Randomized controlled trials (RCT) which evaluated the clinical success of herbal medicine compared with formocresol, ferric sulphate, calcium hydroxide, glutaraldehyde, MTA as a pulpotomy medicament in vital primary molars were included.
Exclusion Criteria
In vitro studies, animal studies, pulpotomies involving permanent teeth, other pulp therapies like indirect pulp capping, direct pulp capping, pulpectomy and studies other than english language were excluded.
Data Extraction and Quality Evaluation
Data extraction for general characteristics of all included studies and variables of outcome were done independently by two investigators. Quality assessment of the included studies and risk of bias was done for all the studies based on five main methodological qualities such as sample size determination, random sequence generation, blinding of personnel and participants, blinding of outcome assessor and allocation concealment. and it was recorded as good, fair and poor according to the criteria shown in [Table/Fig-1]. The studies were assessed and recorded to have a “high risk” of bias if it did recorded “poor” in any one category and “low Risk” of bias if either “good” or “fair” was recorded in all the five categories. For each trial the following data were recorded such as author and year of publication, study design, details of sample size and mean age of participants, details of the Intervention and control used and method of evaluation and details of mean values and outcomes.
Criteria for assessing quality of included studies.
| S.No | Criteria Factor | Description Definition |
|---|
| 1 | Sample size determination | Good: Explanation on how sample size was determined.Fair: Mentioning that sample size was determined without explaining the methodology.Poor: No details on sample size determination. |
| 2 | Random sequence generation | Good: Generated by random numbers or tables, tossed coin, shuffled cards, or any other random sequence generation satisfying consort criteria. In case of restricted randomization, the method used to restrict randomization and the method used for random selection should be specified.Fair: Just the usage of the term randomization or randomly allocated without information of the exact randomization method.Poor: Alternate assignment, case record, number etc. |
| 3 | Blinding of personnel and participants: | Good: The personnel or participant have no knowledge which intervention were given by means of labeling the intervention group as A and B or mention of any other methods.Fair: Just the usage of the word Blinding without information of the exact details.Poor: No information on the blinding of the participants or personnel. |
| 4 | Blinding of Outcome assessor: | Good: Information about the blinding of personnels involved in assessing the outcome of the intervention by means of coding the intervention groups or any other methods.Fair: Just the mention of the term blinding of the outcome assessor without mentioning the method.Poor: No information on the blinding of the outcome assessor. |
| 5 | Allocation concealment | Good: Mention of any method that would could conceal the allocation sequence such that the intervention could not have been foreseen or in advance of or during enrollment, like envelope method, number coded vehicle and central randomization.Fair: Just the mention of allocation concealment has been done without mentioning the method.Poor: No information about allocation concealment. |
Results
Study Inclusions
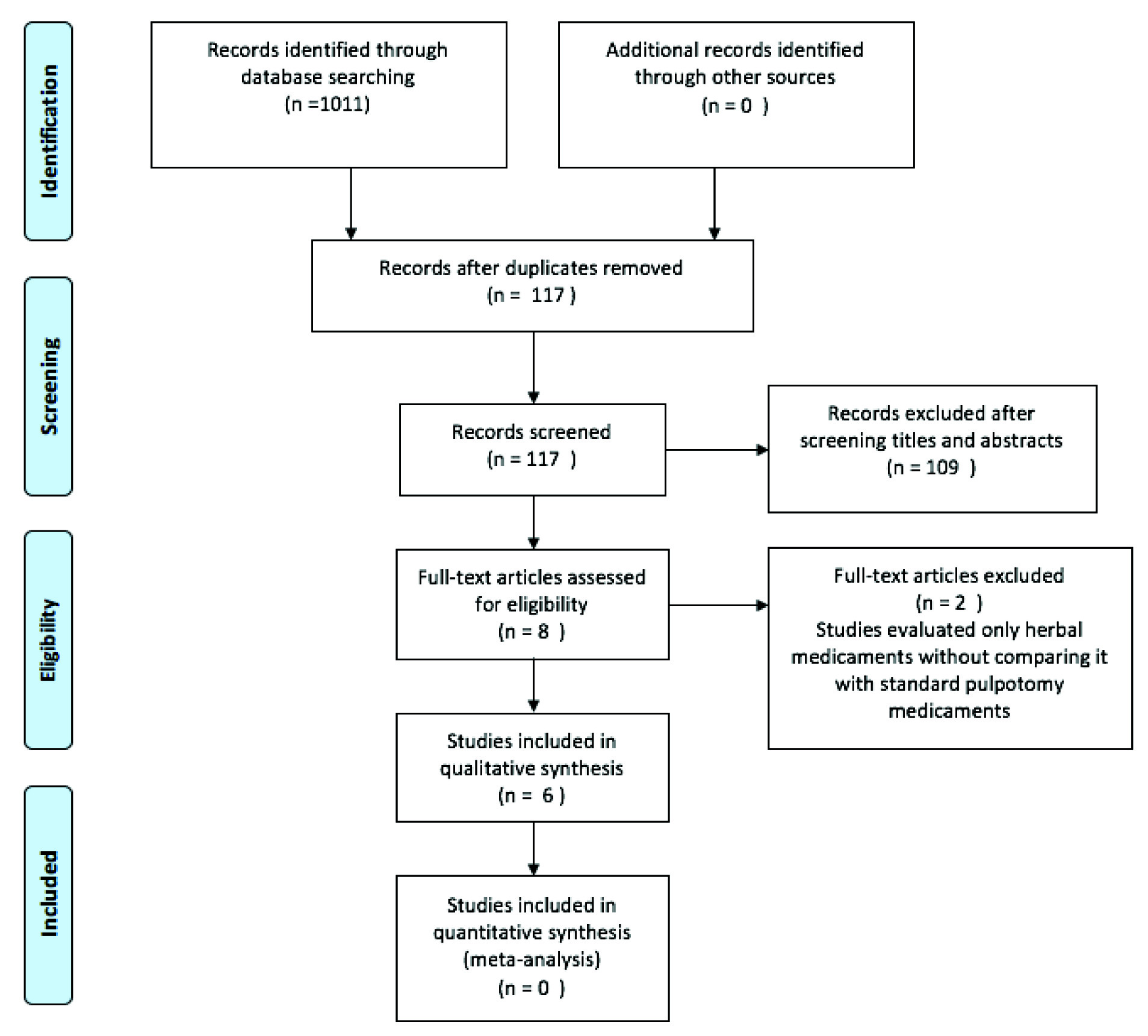
Initial search strategy obtained 1011 relevant articles. Based on screening titles and abstracts, eight articles were included for full text evaluation. Finally, six randomized controlled clinical trials which evaluated the clinical and radiographic success of herbal medicaments compared to gold standard pulpotomy medicaments were retrieved and included in this systematic review [Table/Fig-2] [5,8-12].
PRISMA flow chart illustrating the summmary of search and included studies.
Characteristics of Study Design and Quality of Studies
All of the studies were randomized controlled trials except one study which did not report the study design [5]. All the studies were split mouth clinical trials [5,8-11] except one study [12]. All the studies were published between 2011 to 2015. Four studies were single blinded studies [8-11]. Outcome assessors were blinded. Only one study mentioned about the sample size calculation by power calculation [9]. All studies did not mention about the source of funding. The general information about the characteristics of the selected studies are depicted in [Table/Fig-3].
General characteristics of selected articles.
| Author and year | Study design | Sample size and age | Materials used | Follow up (month) | Afterfollow up | Outcome measurement | Clinicalsuccessrate | Radiographic success rate |
|---|
| Control | Intervention | Control | Intervention | Control | Intervention | Control | Intervention |
|---|
| Odabas ME et al., [10] | Randomized control split moth study | 20 children (male:10,female: 10), 4 to 8 years old | Calcium hydroxide (20) | Ankaferd blood stopper with calcium hydroxide (20) | 1 | 20 | 20 | Clinical and radiographic outcome at 1,3,6,9 and 12 months | 100 | 100 | 100 | 100 |
| 3 | 20 | 20 | 100 | 100 | 100 | 100 |
| 6 | 19 | 20 | 95 | 100 | 95 | 100 |
| 9 | 19 | 19 | 95 | 95 | 90 | 100 |
| 12 | 18 | 19 | 90 | 95 | 90 | 95 |
| Yaman E et al., [8] | Randomized, Single blindclinical trial | 30 patients, 6 to 9 years(17 male, 13 female) | 1:5 dilution of Buckley’s formocreso l(30) | Ankaferd blood stopper | 3 | 30 | 30 | Clinical and radiographic outcome at 3, 6 and 12 months | 100 | 100 | 100 | 100 |
| 6 | 29 | 25 | 100 | 96.7 | 100 | 100 |
| 12 | 28 | 24 | 100 | 100 | 100 | 100 |
| Cantekin K and Gumus H[9] | Randomized Control split mouth study | 35 children, (male 18,female17) 4 to 6 years | Ferric sulphate | Ankaferd blood stopper | 3 | 33 | 33 | Clinical and radiographic outcome at 3, 6,9 and 12 months | 100 | 100 | 100 | 100 |
| 6 | 33 | 33 | 100 | 100 | 100 | 100 |
| 9 | 31 | 30 | 93.9 | 90.9 | 93.9 | 90.9 |
| 12 | 30 | 28 | 90.9 | 84.8 | 87.8 | 84.8 |
| Mohammad SG et al., [5] | Randomized control clinical trial (split mouth design) | 20 patients, 4 to 8 years | Formocresol (20) | Allium Sativum (20) | 6 months | 20 | 20 | Clinical and radiographic outcome at 3 months | 85 | 90 | 85 | 90 |
| Poureslami H et al., [12] | Randomized control clinical trial (split mouth) | 32 patients,(male: 21female: 11) 6 to 10 years, average 7/28 years | 1.5 conc formocresol (32) | Elaegnus Angustifolia (32) | 10 days | | | Measurement of pain by using visual analogue scale. | NR | NR | NR | NR |
| Bharti K et al, [11] | Randomized control clinical trial | 75 children, 3 to 10 years | MTA, biodentine (15 each) | Propolis paste (15) | 0 | 30 | 15 | Clinical and radiographic outcome at 3, 6 and 9 months by means of assigning the scores. | 100 | 100 | 100 | 100 |
| 3 | 30 | 15 | 100 | 100 | 100 | 96 |
| 6 | 30 | 15 | 100 | 100 | 92 | 72 |
| 9 | 30 | 15 | 100 | 100 | 92 | 72 |
Outcome assessment and risk of bias [Table/Fig-4] were assessed based on the consort criteria depicted in the [Table/Fig-1] and all the studies recorded high risk of bias [Table/Fig-4].
| Study | Samplesize determination | Random sequence generation | Allocation concealment | Blindingof participants and personnel | Blinding of outcome assessor | Risk of Bias | Support for Judgement |
|---|
| Odabas M Eet al., [10] | Poor | Fair | Not applicable | Poor | Fair | High | They have not reported the sample size determination. They have mentioned about randomization but method of randomization was not mentioned. The allocation concealment to prevent selection bias was not reported. Blinding of participants and personnel was not reported and outcome assessors were blinded. |
| Yaman E et al., [8] | Poor | Fair | Not applicable | Poor | Good | High | They have not reported the sample size determination or method of randomization of the participants. The allocation concealment to prevent selection bias was not reported. Blinding of participants and personnel is not mentioned and blinding of outcome assessors was done and results were calibrated and interexaminer reliability is tested by using Cohen’s Kappa statistics (cohen’s k=0.75, k=1). |
| Cantekin K and Gumus H [9] | Good | Fair | Not applicable | Poor | Fair | High | They have reported that the sample size determination was done by power calculation at 92% power. Method of randomization of the participants were not mentioned. The allocation concealment to prevent selection bias was not reported. Blinding of participants and personnel is not mentioned and blinding of outcome assessors was done . |
| Mohammad SGet al., [5] | Poor | Poor | Not applicable | Poor | Poor | High | They have not reported the sample size determination or method of randomization of the participants. The allocation concealment to prevent selection bias was not reported. Blinding of participants and personnel and outcome assessors was not reported. |
| Poureslami Het al., [12] | Poor | Good | Not applicable | Poor | Poor | High | They have not reported the sample size determination. They have mentioned about method of randomization of the participants by table of random numbers. Allocation concealment to prevent selection bias was not reported. Blinding of participants and personnel and outcome assessors was not reported. |
| Bharti Ket al., [11] | Poor | Good | Poor | Poor | Good | High | They have not reported the sample size determination. They have mentioned about randomization but method of randomization was not mentioned. The allocation concealment to prevent selection bias was not reported. Blinding of participants and personnel was not reported and outcome assessors were blinded. Interexaminer reliability is tested by using Cohen’s Kappa statistics (cohen’s k=0.75, k=1). |
Discussion
The purpose of this paper was to investigate the clinical and radiographic success rates of herbal pulpotomy medicaments to substantiate its effectiveness as a substitute to standard pulpotomy medicaments. The articles included in this systematic review evaluated various herbal medicinal products like allium sativum, elaegnus angustifolia, ankaferd blood stopper and propolis as a pulpotomy medicament compared to standard pulpotomy medicaments. All the studies [5,8-11] assessed the clinical and radiographic parameters like tenderness to percussion, postoperative pain, presence of sinus tract and mobility, presence of periodontal ligament widening, external or internal root resorption, furcal or periapical radiolucencies and pulp canal obliteration of different herbal agents compared to the standard pulp dressing medicaments. Poureslami H et al., evaluated the clinical success by measuring the degree of pain by using visual analogue scale during 10 days after the treatment by means of the questionnaire [12].
(i) Allium sativum is commonly known as garlic and is used to treat many infectious diseases due to its potent antimicrobial property because of the presence of allicin produced by enzyme allicinase which is active against a wide spectrum of bacteria [13]. Martin KW and Ernst E reported that garlic inhibits various Gram-positive and Gram-negative bacteria [14] including multidrug resistant strains of Streptococcus mutans isolated from human carious teeth [15]. It also has antiviral and antifungal property [16]. Mohammad SG et al., compared the success rates of Allium sativum oil (Captin company, CAP pharm and formocresol as a pulpotomy medicament by placing the medicament dampened with cotton pellet for 5 minutes over the pulp stumps and found that the overall clinical success after 6 months for Allium sativum was 90% compared to that of 85% with formocresol [5].
(ii) Elaegnus angustifolia fruit is also known as a Russian olive. It is a potent antinflammatory agent due to the antioxidant properties of flavenoids and terpenoids. It also has analgesic, antipyretic and coagulation properties [17]. It is not available commercially, but naturally fresh Elaegnus angustifolia fruits are picked from the tree and dried. The seeds of fruits are separated and pure powder is prepared and then the prepared powder is sterilized to be used as a pulpotomy medicament. Poureslami H et al., assessed the degree of pain during 10 days after treatment with Elaegnus angustifolia fruit and formocresol by using the soft paste mixture prepared by adding 15 mg of Elaegnus angustifolia powder and 0.9% sodium chloride solution, which was placed on orifices in 1 mm thickness and found that the pain was decreased significantly in both the groups but the decrease in the formocresol group was more than in the Elaegnus angustifolia group [12].
(iii) Ankaferd Blood Stopper (ABS) is made of five plants namely Thymus vulgaris, Glycyrrhiza glabra, Vitis vinifera, Alpinia officinarum and Urticadioica and has widely been used as a haemostatic agent in pulpotomy due to its rapid formation of a protein network and erythrocyte aggregation [18]. ABS is a potent antibacterial agent [19]. Three studies have compared ABS as a pulpotomy material. Yaman E et al., used ABS solution (Ankaferd Health Products Ltd., Istanbul, Turkey) and applied it with a moistened cotton pellet for 15 seconds and found 85.7% clinical and radiographic success compared to formocresol (89.3%). But, there was no statistical significant difference between the groups at 3, 6 and 12 month follow up [8]. Cantekin K and Gumus H also evaluated the clinical and radiographic success rates of ABS and ferric sulphate. At 12 month follow up, the clinical and radiographic success rates of ferric sulphate was approximately 90.9% and 87.8% respectively and for ABS group it was 84.8%. Teeth treated with ABS showed a slightly lower success rate than those treated with ferric sulphate, but was not statistically significant [9]. Odabas ME et al., reported that the total success rate of calcium hydroxide with ABS after 12 months was 95% and calcium hydroxide group showed 90% success. There was no statistically significant difference between the groups. Internal resorption was the most common failure in both the groups [10]. Calcium hydroxide was the first medicament that formed reparative dentin bridge in pulpotomies [20]. Bystrom A et al., stated that it has good antibacterial property [21]. The usage of calcium hydroxide is not used routinely for primary dentition because it produces internal root resorption [21,22].
(iv) Propolis, has gained popularity in dentistry as an intracanal medicament, as a cariostatic agent, for storage of avulsed teeth and also as a root canal irrigant due to its superior antibacterial, anti-inflammatory and immunomodulating properties [23]. The antibacterial activity of propolis is due to flavonoids and aromatic acids and esters present in resin [24]. Propolis helps in collagen synthesis and assists in wound healing. It was reported that propolis showed least inflammation and greater fibrous tissue formation, maintained pulp vitality complete calcific bridge was formed. Hence, propolis has potential to be used as a pulpotomy agent [25].
Bharti K et al., compared MTA, biodentine and propolis. About 1.5 g of propolis extract powder (HI-Tech Natural Products Ltd., New Delhi, India) at 100% was mixed with 1.75 ml of polyethylene glycol and placed over the pulp stumps to a thickness of 2 mm-3 mm. They found that the clinical and radiographic success was more favourable for MTA and biodentine compared to propolis at 9 months follow up [11].
The included studies have shown that the clinical and radiographic success rates of herbal medicine such as Allium sativum, ABS, Elaegnus angustifolia and propolis were similar in comparison with the standard pulpotomy medicament. But all the studies recorded high risk of bias on quality assessment. Herbal medicines can be used as a substitute to standard pulpotomy medicaments in clinical situations, but high quality evidence is required.
Limitation
Limitation of this systematic review is that it includes only articles published in English and due to the heterogeneity of the included studies; meta-analysis was not possible to substantiate the effectiveness of superior herbal pulpotomy medicaments. Commercial availability of one of the herbal pulpotomy medicament implementing Elaegnus angustifolia is not available, but it is prepared naturally as a fresh extract and is used during the pulpotomy procedure.
Conclusion
Based on this available evidence, it is difficult to determine the success rates of herbal pulpotomy medicaments. The overall quality of research in the clinical success of herbal medicine as a pulpotomy medicament is not adequate. More high quality studies with proper methodological designs and long term follow ups are required to find a suitable substitute to replace standard pulpotomy medicaments in primary teeth.