Materials and Methods
The present invitro study was carried out and completed within the month of January of 2017 after obtaining clearance from the Ethical Clearance Committee of Sharavathi Dental College and Hospital, Shimoga, India. (Clearance number: SDC/SMG/2016/722/A) For the purpose of this study, stock cultures of A.actinomycetemcomitans (ATCC No. - 43718), P.gingivalis (ATCC No. - 33277, 53978), P.intermedia (ATCC No. - 25611), S.mutans (ATCC No. - 25175) and F. nucleatum (ATCC No. - 25586) (from Himedia laboratories) were obtained from the Department of Microbiology, Nathaji Rao G Halgekar’s Institute of Dental Sciences and Research Center, Belgaum, India. Chlorhexidine 0.2% mouthrinse (Warren Pharma) was used as a positive control for the study.
Fine marigold flakes were procured from Aum Agri Freeze Foods, Baroda, India. Flakes were dissolved in dimethyl sulfoxane to obtain a crude form for ease of use in the experiments [13,14].
Minimum Inhibitory Concentration (MIC) Procedure
MICs are defined as the lowest concentration of an antimicrobial that will inhibit the visible growth of a microorganism after overnight incubation or 24 hour incubation. Microbroth dilution method was used to determine the MIC.
A 200 μL of Brain Heart Infusion (BHI) broth was added into the 9 tubes separately. For drug dilution procedure (using the microbroth dilution method) 9 dilutions of chlorhexidine and Calendula officinalis were prepared with BHI. An initial tube contained 20 μL of the test agent to which 380 μL of BHI broth was added. To start the dilution process, 200 μL from the initial tube (tested agent and BHI) was transferred to the first tube containing 200 μL of BHI broth. This was considered as 101 dilution. From 101 diluted tube, 200 μL was transferred to the next (second) tube to make it 102 dilution. The serial dilution was repeated up to 109 dilution for each tested agent. About 5 μL from the maintained stock culture of each organism, was taken and added into 2 mL of BHI broth and made into a suspension and the turbidity was adjusted with reference to the standard 0.5 McFarland unit such that the inoculum would contain 1×108 CFU/mL. Turbidity was evaluated after incubating for 24 hours [15].
The test was interpreted as follows: Clear broths were interpreted as the target organism being visibly sensitive to the drug (no growth); turbid broths were interpreted as the target organism being visibly resistant (growth is apparent) to the tested agent.
Minimum Bactericidal Concentration (MBC) Procedure
To determine bactericidal concentration, the MIC dilution tubes with no visible growth (no turbidity) and the control tube were subcultured onto the respective media (Brain Heart Infusion agar for A.actinomycetemcomitans and blood agar for the remaining microbes) and incubated for 24 hours anaerobically at 37°C and the colonies were counted the next day. The organisms’ growths from the control tubes were then compared with the organism growth from the MIC test tubes.
Inoculum Preparations
The colonies were transferred from the plates to the BHI broth with a sterilized straight nichrome wire. The turbidity was visually adjusted with BHI broth to equal that of a 0.5 McFarland unit turbidity standard that has been freshly prepared [16].
The test was interpreted as follows: If there were similar number of colonies it indicated bacteriostatic activity only, while reduced number of colonies denoted a partial or slow bactericidal activity. No growth was observed if the whole inoculum has been killed.
Agar Well Diffusion Procedure
Well plate method was used for agar diffusion procedure. After adjusting the inoculum to a 0.5 McFarland unit turbidity standard, a sterile cotton swab was dipped into the inoculum and rotated against the wall of the tube above the liquid to remove excess inoculum. Entire surface of the blood agar plate was swabbed three times, rotating plates approximately 60° between streaking to ensure even distribution. The inoculated plate was allowed to stand for at least 3-15 minutes before punching the wells in agar plate.
A heated hollow tube of 5 mm diameter was pressed on the inoculated agar plate and removed immediately to punch wells. Five such wells were made on each plate. Two such plates for each organism were prepared. A 50 μL/mL of C.officinalis was added into the respective wells on each plate. The plates were incubated within 15 minutes of drug application for 18-24 hours at 37°C anaerobically. The plates were read only if the lawn of growth was confluent or nearly confluent. The diameter of the inhibition zone was measured to the nearest whole millimeter by using calipers. Two plates for each organism were prepared. A zone of 12 mm was considered the reference zone of inhibition above which test microbes were considered sensitive and below which they were considered resistant to the test drugs [17].
Time Kill Curve
The dilutions were done in a manner similar to that of MIC. The broth (BHI, 100 μL volume) and the test compounds were mixed in equal proportions and inoculated with the test microbes. The tubes were then placed in a CO2 jar and the baseline was noted at 0 hours. At every 5 minutes, 10 minutes, 30 minutes and 2 hours the mixture was cultured or plated. Incubation was done according to growth requirement, i.e., in CO2 anaerobic jar. After 48-78 hours of incubation, the plates were removed and the colonies were counted [17]. Simple statistics was used for the calculations.
Results
Minimum Inhibitory Concentration
A.actinomycetemcomitans as well as F.nucleatum was sensitive to C.officinalis at 100 μg/mL and was resistant at all other concentrations [Table/Fig-1]. On the other hand MIC of C.officinalis at 0.8 μg/mL for P.gingivalis [Table/Fig-2] as well as P.intermedia (50 μg/mL) was lower than chlorhexidine which was at 1.6 μg/mL [Table/Fig-3]. C.officinalis MIC for S.mutans was at 3.12 μg/mL as compared to chlorhexidine which inhibited growth at 6.25 μg/mL.
Minimum Inhibitory Concentration.
| Quantity (μg/mL) | 100 | 50 | 25 | 12.5 | 6.25 | 3.12 | 1.6 | 0.8 | 0.4 | 0.2 |
|---|
| A.actinomycetemcomitans |
| Chlorhexidinedigluconate 0.2% | S* | S | S | S | R† | R | R | R | R | R |
| C.officinalis | S | R | R | R | R | R | R | R | R | R |
| P.gingivalis |
| Chlorhexidinedigluconate 0.2% | S | S | S | S | S | S | S | R | R | R |
| C.officinalis | S | S | S | S | S | S | S | S | R | R |
| P.intermedia |
| Chlorhexidinedigluconate 0.2% | S | S | R | R | R | R | R | R | R | R |
| C.officinalis | S | S | S | S | S | S | S | S | R | R |
| F.nucleatum |
| Chlorhexidinedigluconate 0.2% | S | S | R | R | R | R | R | R | R | R |
| C.officinalis | S | R | R | R | R | R | R | R | R | R |
| S.mutans |
| Chlorhexidinedigluconate 0.2% | S | S | S | S | S | R | R | R | R | R |
| C.officinalis | S | S | S | S | S | S | R | R | R | R |
*Sensitive,†Resistant
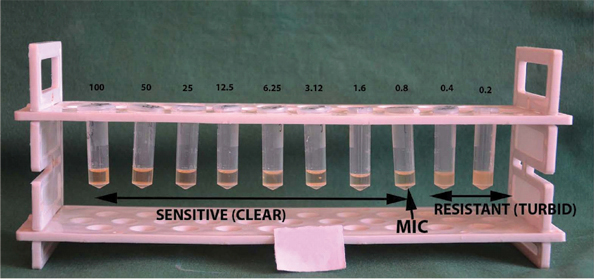
Minimum Inhibitory Concentration (MIC) of Calendula officinalis against P.gingivalis.
*Turbidity observed in tubes containing 0.4 and 0.2 μg/mL of broth indicating resistance. Clear broth observed in all the concentrations above 0.4 μg/mL indicating sensitivity to C.officinalis. Arrow indicates minimum inhibitory concentration of 0.8 μg/mL of chlorhexidine against P.gingivalis.
Note: Concentration expressed in μg/mL.
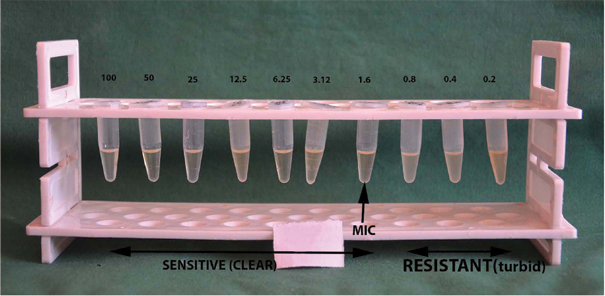
Minimum Inhibitory Concentration (MIC) of chlorhexidine 0.2% against P.gingivalis.
*Turbidity observed in tubes containing 0.8, 0.4 and 0.2 μg/mL of the broth, indicating resistance. Clear broth observed in all the concentrations above 0.8 μg/mL, indicating sensitivity to chlorhexidine 0.2%. Arrow indicates MIC of 1.6 μg/mL of chlorhexidine against P.gingivalis.
Note: Concentration expressed in μg/mL
Minimum Bactericidal Concentration
C.officinalis failed to inhibit the growth of P.gingivalis as well as P.intermedia at any concentration, while 6.25 μg/mL of chlorhexidine 0.2% turned out to be bactericidal for both P.gingivalis and P.intermedia. C.officinalis was bactericidal to A.actinomycetemcomitans at 100 μg/mL, to S.mutans at 6.25 and was otherwise not bactericidal to other microbes tested. Chlorhexidine was bactericidal to both A.actinomycetemcomitans and F.nucleatum at 6.25 μg/mL. For S.mutans MBC was identical for both test drugs at 6.25 μg/mL [Table/Fig-4].
Minimum bactericidal concentration.
| Quantity of drug(ug/mL) | 0.2 | 0.42 | 0.8 | 1.6 | 3.12 | 6.25 | 12.5 | 25 | 50 | 100 |
|---|
| P.gingivalis |
| Chlorhexidinedigluconate 0.2% | 120 | 100 | 80 | 50 | 30 | NG | NG | NG | NG | NG |
| C.officinalis | 250 | 230 | 200 | 180 | 150 | 130 | 130 | 120 | 110 | 100 |
| P.intermedia |
| Chlorhexidine digluconate 0.2% | 200 | 180 | 170 | 150 | 80 | NG | NG | NG | NG | NG |
| C.officinalis | 300 | 280 | 250 | 230 | 200 | 150 | 130 | 110 | 80 | 50 |
| A.actinomycetemcomitans |
| Chlorhexidine digluconate 0.2% | 300 | 270 | 250 | 230 | 180 | NG | NG* | NG | NG | NG |
| C.officinalis | 300 | 290 | 280 | 260 | 230 | 200 | 180 | 150 | 120 | NG |
| F.nucleatum |
| Chlorhexidine digluconate 0.2% | 300 | 260 | 230 | 180 | 150 | NG | NG | NG | NG | NG |
| C.officinalis | 300 | 280 | 225 | 200 | 150 | 130 | 120 | 110 | 100 | 80 |
| S.mutans |
| Chlorhexidine digluconate 0.2% | 180 | 120 | 80 | 50 | 50 | NG | NG | NG | NG | NG |
| C.officinalis | 200 | 180 | 150 | 100 | 10 | NG | NG | NG | NG | NG |
*NG-No Growth
Agar Well Diffusion Method
Both the agents were poured in the wells [Table/Fig-5] and resultant zones of inhibition were noted. A.actinomycetemcomitans and P.gingivalis were considerably more sensitive to C.officinalis than to chlorhexidine. The other organisms exhibited more sensitivity towards chlorhexidine than to calendula. F.nucleatum was the least sensitive to chlorhexidine amongst all the organisms tested [Table/Fig-6].
Agar Well Diffusion- Wells punched deposited with 50 μL of the test drugs: a) C.officinalis; and b) Chlorhexidine.
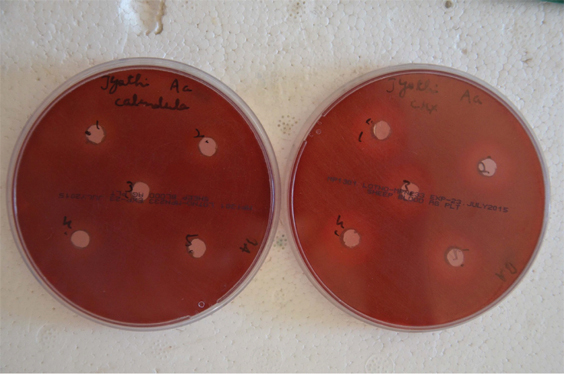
| Quantity Added (μL) | 50 | 50 | 50 | 50 | 50 |
|---|
| P.intermedia |
| Chlorhexidinedigluconate 0.2% | 20 mm* | 20 mm | 20 mm | 20 mm | 20 mm |
| C.officinalis | 15 mm | 10 mm | 10 mm | 10 mm | 10 mm |
| P.gingivalis |
| Chlorhexidinedigluconate 0.2% | 20 mm | 20 mm | 20 mm | 20 mm | 20 mm |
| C.officinalis | 22 mm | 22 mm | 22 mm | 22 mm | 22 mm |
| A.actinomycetemcomitans |
| Chlorhexidinedigluconate 0.2% | 13 mm | 13 mm | 13 mm | 13 mm | 13 mm |
| C.officinalis | 20 mm | 20 mm | 20 mm | 20 mm | 20 mm |
| S.mutans |
| Chlorhexidinedigluconate 0.2% | 20 mm | 20 mm | 20 mm | 20 mm | 20 mm |
| C.officinalis | 15 mm | 15 mm | 15 mm | 15 mm | 15 mm |
| F.nucleatum |
| Chlorhexidinedigluconate 0.2% | 18 mm | 18 mm | 18 mm | 18 mm | 18 mm |
*Zone of inhibition of growth of microorganisms measured in millimeters
Time Kill Curve Method
Chlorhexidine exhibited lethality at 5 minutes for all periodontal pathogens. C.officinalis exhibited lethality against A.actinomycetemcomitans at 30 minutes [Table/Fig-7] and P.intermedia at 30 minutes, but failed to be bactericidal to P.gingivalis at any time interval, however, decreasing colony forming units were observed with increasing time intervals. C.officinalis showed lethality to F.nucleatum at 2 hours and to S.mutans at 10 minutes as against chlorhexidine at 5 minutes and at 0 minutes respectively [Table/Fig-8].
Time kill curve- a) Colony Forming Units (CFU log10) counted 72 hours later. Growth observed at 0, 5 and 10 minutes and no growth at 30 minutes and 2 hours for C.officinalis; b) Colony Forming Units of 110 (CFU log10) counted 72 hours later. Growth observed at 0 minutes and no growth at other time intervals for chlorhexidine.
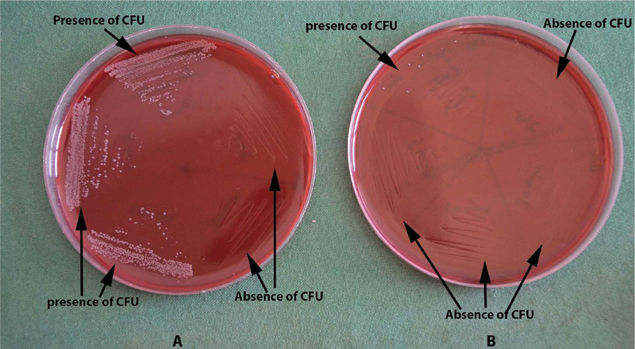
| Time | 0minute | 5minutes | 10minutes | 30minutes | 2hours |
|---|
| A.actinomycetemcomitans |
| Chlorhexidine digluconate 0.2% | 110 | NG* | NG | NG | NG |
| C.officinalis | 180 | 165 | 130 | NG | NG |
| P.gingivalis |
| Chlorhexidine digluconate 0.2% | 120 | NG | NG | NG | NG |
| C.officinalis | 120 | 110 | 90 | 70 | 55 |
| P.intermedia |
| Chlorhexidine digluconate 0.2% | 40 | NG | NG | NG | NG |
| C.officinalis | 90 | 70 | 40 | NG | NG |
| S.mutans |
| Chlorhexidine digluconate 0.2% | NG | NG | NG | NG | NG |
| C.officinalis | 20 | 10 | NG | NG | NG |
| F.nucleatum |
| Chlorhexidine digluconate 0.2% | 50 | NG | NG | NG | NG |
| C.officinalis | 150 | 140 | 130 | 90 | NG |
*NG-No Growth
Discussion
A.actinomycetemcomitans was found to be fairly sensitive to C.officinalis, almost on par with chlorhexidine in its ability to inhibit or kill A.actinomycetemcomitans. While MIC of C.officinalis for A.actinomycetemcomitans in this study was a high 100 μg/mL it contrasted with an MIC of as low as 25 μg/mL in another study [18]. C.officinalis however exhibited a 20 mm zone of inhibition as against a 13 mm of chlorhexidine against A.actinomycetemcomitans. In another study that compared the antimicrobial activity (against A.actinomycetemcomitans and P.gingivalis) of a hydroalcoholic extract of C.officinalis along with a host of other plant products and chlorhexidine 0.12%, the MIC and MBC of C.officinalis against A.actinomycetemcomitans was far higher than chlorhexidine 0.12% [19].
The solvent used to make C.officinalis preparation in this study was dimethyl sulfoxide, certain other studies have used methanolic extracts of C.officinalis and it was found to be effective against even Multi Drug Resistant Pathogens (MDR pathogens) [12]. Methanolic extraction of C.officinalis enables it to have a higher content of polyphenols and antioxidants. However, we have used C.officinalis in an aqueous solution dissolved in dimethyl sulfoxide. C.officinalis in combination with a few other herbs was tested against A.actinomycetemcomitans in a pilot study. There was no significant reduction in plaque or gingival index neither was there a significant reduction in A.actinomycetemcomitans [20], yet this warrants further studies with larger sample size of volunteers.
P.gingivalis has shown a few conflicting results against C.officinalis. C.officinalis exhibited an MIC of 0.8 μg/mL which was slightly better than chlorhexidine at 1.6 μg/mL and yet did not show lethality against P.gingivalis at any concentration in the sub culturing, but exhibited a considerably high zone of inhibition of 22 mm in agar well diffusion. It needs to be noted here that agar well diffusion method does have a few limitations, such as the issue pertaining to the type of antibiotic used in the test and its break points etc., [21] or its inability to test for susceptibility when it comes to certain fastidious micro-organisms [22]. C.officinalis is rich in polyphenols, while certain other herbs, also rich in polyphenols, are known to be specifically inhibitory to virulence factors of P.gingivalis [23], no such data exists for C.officinalis. In this study, C.officinalis failed to be bactericidal to P.gingivalis even at two hours in the time kill curve despite having a lower MIC than Chlorhexidine and relatively high zone of inhibition of all the microbes tested in agar well diffusion. In another study involving chlorhexidine 0.12%, C.officinalis and other herbs, C.officinalis exhibited a significantly lower MIC against P.gingivalis than against A.actinomycetemcomitans [19].
P.intermedia, a Gram-negative, obligate anaerobic pathogenic bacterium, was considerably sensitive to C.officinalis at an MIC of 0.8 μg/mL as against 50 μg/mL of chlorhexidine. However, C.officinalis failed to exhibit total lethality to P.intermedia at any concentration despite reduction in colony forming units. C.officinalis has exhibited considerable anti-microbial activity against P.intermedia in an earlier study [24].
Socransky and Haffajee’s study compared chlorhexidine, listerine and an herbal mouthrinse containing C.officinalis against about 40 oral micro organisms. C.officinalis exhibited an MIC of 16 μg/mL for P.intermedia which was significantly high compared to the MIC in this study. However, mouthrinse used in their study, contained other herbal extracts and MIC dilution tube preparation protocol also differed from our study [25].
S.mutans, a primary aetiologic agent of human dental caries, is particularly effective at forming biofilms on the hard tissues of the human oral cavity. It adheres to primary colonizers by cell-to-cell interaction. S.mutans’ cariogenicity is well known and some of it is attributed to its property of adhesion to tooth surface or pellicle [26]. Several herbal products have been shown to be effective against S.mutans. Green tea mouthrinse for example, has shown significant antimicrobial activity against cariogenic micro organisms like S.mutans and Lactobacillus [27]. Garlic with lime has also shown promise with in vitro antimicrobial activity against S.mutans [28]. In the present study, C.officinalis showed significant antimicrobial activity against S.mutans, with a lower MIC than chlorhexidine and fewer colonies of S.mutans were observed for C.officinalis at a dilution identical to chlorhexidine and yet zone of inhibition was much smaller as compared to chlorhexidine. C.officinalis in the form of tincture (in varying dilutions) has shown considerable antimicrobial activity against S.mutans in a study that compared it with another herbal extract and chlorhexidine 0.12%. The zones of inhibition ranged from 0-12.5 mm as against chlorhexidine which exhibited upto 14 mm [29] which is again similar to our study where chlorhexidine had higher zones of inhibition than C.officinalis against S.mutans. Similarly, when C.officinalis was compared with chlorhexidine 0.12% and Listerine it was observed that C.officinalis had a far lower MIC than listerine against S.mutans but higher MIC compared to chlorhexidine [25].
All studies have slightly different protocols for preparation C.officinalis, therefore large differences in MIC should be viewed keeping in mind the different constituents, protocols, vehicles and concentrations that make each study design different despite using the same plant extract.
C.officinalis was completely ineffective against F.nucleatum, a nonmotile, and gram negative, anaerobic micro-organism, at all concentrations when tested for MBC. However, with C.officinalis, it does seem like longer the drug is in contact, the more effective its antimicrobial activity is, as exhibited by the antimicrobial activity at two hours. Time kill curve data for a potential antimicrobial agent becomes important in terms of the target micro-organisms it is intended to have antimicrobial activity against. If the target microbes (periodontal pathogens in this instance) have an average regrowth period of few minutes while the potential drug possesses a time kill curve of two hours, it is not desirable irrespective of what the minimum inhibitory or bactericidal concentration is. C.officinalis also did not fare well in another study where it was used as a methanolic extract. It exhibited an extremely high MIC against F.nucleatum as compared to the other organisms in this particular study [30].
In the present study, C.officinalis was considerably effective against S.mutans, an organism that relies on adhesion to tooth surface as one of its virulence factors [31]. Cranberry juice, because of its high molecular polyphenol content is able to inhibit the adhesion of S.mutans [32] similar to it’s the antiadhesive action against E.coli in preventing urinary tract infection in women [33]. Also, polyphenols of Myrothamnus flabellifolia were shown to reduce P. gingivalis adhesion and invasion upto about 50% by interacting with certain proteins that were specific to P.gingivalis [34]. Since, polyphenols are one of the chief constituents of C.officinalis, its antimicrobial potential and anti adhesive properties could as well translate to anti-plaque applications in vivo, further studies are required to confirm the same. If C.officinalis has to prove its worth against other key periodontal pathogens, animal model studies have to precede safety and efficacy studies in human clinical trials.
Limitation
All procedures were carried out only once, hence relevant analysis for accurate determination of efficacy could not be done.
Conclusion
This study exhibited the significant antibacterial effect C.officinalis had, on 4 major periodontal pathogens and S.mutans. However, its efficacy was most evident against A.actinomycetemcomitans, and least against F.nucleatum. Even though there is data on C.officinalis being incorporated in an antimicrobial mouthwash along with other herbal agents, it still needs to be investigated as a stand alone antimicrobial agent. More in vivo studies in different carrier vehicles need to be carried out to assess its longevity and feasibility in oral hygiene maintenance applications as well as locally delivered agent in a periodontal pocket. The key components/molecules in C.officinalis that are responsible for its antimicrobial efficacy still remain unexplored. Studies involving multiple repetitions of the tests as well against more number of micro-organisms would be the next step if C.officinalis has to be included into mainstream periodontal anti-infective therapy.