An Unusual Presentation of Metanephric Adenofibroma: A Rare Case Report
Kiran Agarwal1, Jyoti Garg2, Gunjan Mahajan3, Partap Singh Yadav4, Divya Manohar5
1 Director Professor, Department of Pathology, Lady Hardinge Medical College and Associated Hospitals, New Delhi, Delhi, India.
2 Senior Resident, Department of Pathology, Lady Hardinge Medical College and Associated Hospitals, New Delhi, Delhi, India.
3 Senior Resident, Department of Pathology, Lady Hardinge Medical College and Associated Hospitals, New Delhi, India.
4 Assistant Professor, Department of Paediatric Surgery, Lady Hardinge Medical College and Associated Hospitals, New Delhi, India.
5 Postgraduate Student, Department of Pathology, Lady Hardinge Medical College and Associated Hospitals, New Delhi, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Jyoti Garg, 40-C, MIG, DDA Flats, Rani Jhansi Complex, Motia Khan, New Delhi-110055, India.
E-mail: drjyotigarg1705@gmail.com
Metanephric adenofibroma is a rare renal neoplasm with only a few case reports in literature. In majority of cases, it is asymptomatic. However, it may present with haematuria, polycythemia or hypertension. Radiologically, it is indistinguishable from other solid renal tumours. Definitive diagnosis can only be made on the basis of histopathology. It is a benign neoplasm and requires only surgical excision with no need for chemotherapy. Involvement of urinary bladder and presentation as bladder mass has never been reported. In this case report, we present a case of metanephric adenofibroma in a two-year-old male child manifesting with haematuria and urinary bladder mass.
Biphasic renal tumour, Haematuria, Paediatric renal neoplasm, Urinary bladder mass
Case Report
A two-year-old male child presented with haematuria and fever for six months. Patient was a resident of a rural area in a neighboring state and was being treated by a private medical practitioner there. He was being treated for urinary tract infection with antipyretics and antibiotics. A urinary examination report showed haematuria. However, it was not investigated further. After four months, an ultrasound was done, the findings of which have been mentioned. However, no further workup was done and the child was finally brought to our hospital six months from onset of symptoms.
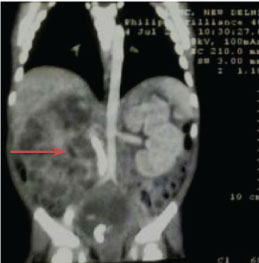
On general clinical examination, the child was found to be irritable and febrile with pallor. No other significant clinical finding was seen. An ultrasound done two months back showed enlarged right kidney with dilated pelvicalyceal system. It also showed a 22 mm X 14 mm isoechoic tubular lesion projecting from right vesicoureteric junction into urinary bladder. Provisional diagnosis was made in lines of a blood clot or a mass lesion. Computerized tomography of abdomen [Table/Fig-1] showed markedly distended urinary bladder with almost whole of the lumen occupied by a heterogeneously enhancing lesion. The lesion also extended into the prostatic urethra. Right kidney was found to be enlarged (108 mm x 51 mm) with an ill-defined heterogeneously enhancing lesion involving the medullary collecting system and pelvicalyceal system causing marked parenchymal thinning. Right renal pelvis and uppermost ureter appeared markedly thickened. Left kidney was normal in size and showed mild hydronephrosis with dilated pelvis and upper ureter, possibly secondary to bladder mass. Based on these radiological findings, a possibility of a malignancy arising from urinary bladder (rhabdomyosarcoma) was suggested.
CT scan abdomen: enlarged right kidney with ill-defined heterogeneously enhancing lesion involving medullary collecting system and pelvicalyceal system.
A small incisional biopsy was taken from urinary bladder mass and sent for histopathological examination. Microscopic examination of H&E stained sections showed a polypoid tumour composed predominantly of uniform spindle cells embedded in loose myxoid stroma. Small foci of epithelial component in the form of tubules and glomeruloid structures were also noted [Table/Fig-2]. No cytological atypia, mitotic activity or necrosis was seen. No blastemal component, cambium layer or rhabdomyoblasts were seen. On immunohistochemistry, the spindle cells were positive for vimentin and negative for myogenin. Epithelial component was WT1(Wilms’ Tumour) positive. Based on these histological features, rhabdomyosarcoma was ruled out and a possibility of botryoid Wilms’ tumour was suggested.
Bladder mass biopsy: Tumour composed of uniform spindle cells embedded in loose myxoid stroma with small foci of epithelial component (H&E; 10X).
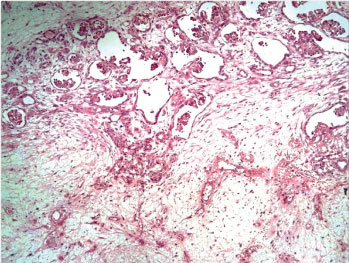
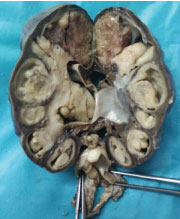
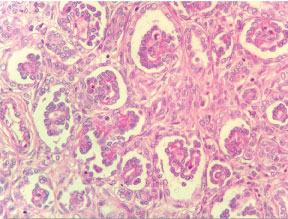
The patient then underwent right nephroureterectomy along with excision of urinary bladder mass. Gross examination of nephrectomy specimen showed enlarged kidney with bosselated external surface. Serial sectioning showed a solid, fleshy, grey white growth involving the dilated pelvicalyceal system and renal medulla with compressed cortex at the periphery. The tumour was seen protruding into renal pelvis and ureteric lumen [Table/Fig-3]. Separately received urinary bladder mass was similar in appearance as tumour in kidney. Histopathological examination of sections from kidney showed a wellcircumscribed, unencapsulated, biphasic tumour comprising of epithelial and stromal components. Epithelial element was composed of closely packed tubules and glomeruloid structures. Tubules were lined by uniform cells having round to oval nuclei with delicate chromatin, indistinct nucleoli and clear to eosinophilic cytoplasm. Some tubules showed papillary infoldings, producing a glomeruloid appearance [Table/Fig-4], many psammoma bodies were also noted. Stromal component showed hypocellular and hypercellular areas, comprising of spindle cells in loose myxoid stroma. Spindle cells showed thin hyperchromatic nuclei with slender indistinct cytoplasmic extensions. At places, stromal cells were seen to be concentrically arranged around epithelial components [Table/Fig-5]. Angiodysplasia of vessels was noted in the form of epithelioid transformation of medial smooth muscle cells of intratumoural arterioles [Table/Fig-6]. No cytological atypia, mitotic activity and necrosis were seen. No blastemal component was seen in the multiple sections examined. Histopathological examination of bladder mass showed similar morphology. On immunohistochemistry, the epithelial component was positive for Cytokeratin (CK), Epithelial Membrane Antigen (EMA) and WT1. Focal CK7 positivity was also seen. Stromal cells showed positivity for vimentin and CD34 [Table/Fig-7]. Ki67 index was less than 1%. Based on these features, a final diagnosis of metanephric adenofibroma was made. The patient was closely followed up for nine months and there were no postoperative complications or recurrence.
Gross examination of nephrectomy specimen: solid grey white tumour involving pelvicalyceal system and protruding into ureter.
Epithelial component of tumour: tightly packed tubules and glomeruloid structures (H&E, 40X).
Stromal component of tumour: spindle cells showing concentric cuffing around epithelial element (H&E, 40X).
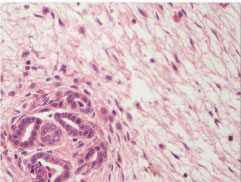
Angiodysplasia: epithelioid transformation of medial smooth muscle cells (H&E, 40X).
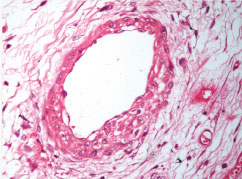
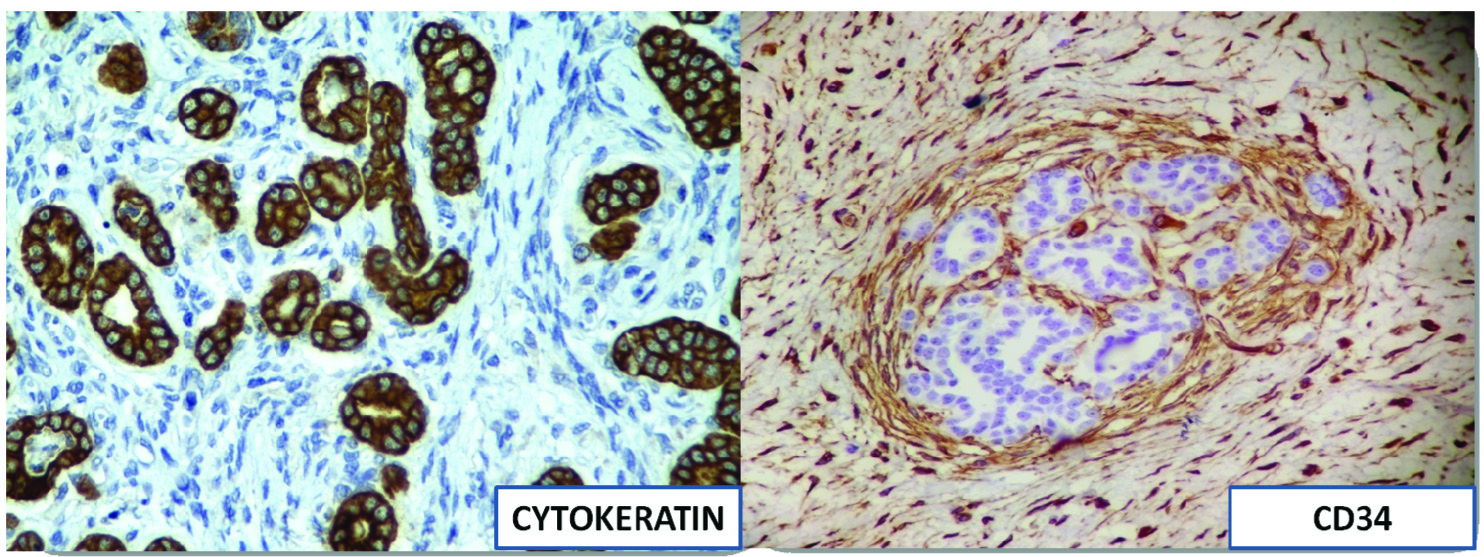
Immunohistochemistry: epithelial component cytokeratin positive and stromal component CD34 positive (cytokeratin and CD34, 10X).
Discussion
Metanephric adenofibroma is a rare renal tumour, first described by Hennigar RA and Beckwith JB, in 1992 [1]. Since then, there have been a few case reports in literature [2-6]. It was first thought to be highly differentiated Wilms’ tumour. However, now it is considered to be a tumour lying within the spectrum of metanephric neoplasms, which include metanephric adenoma and metanephric stromal tumour at the two ends. While metanephric adenoma is a purely epithelial tumour and metanephric stromal tumour a purely stromal lesion, metanephric adenofibroma is a biphasic tumour consisting of both elements in varying proportions [3].
Metanephric adenofibroma is a benign tumour affecting mainly young individuals [1]. Patients range in age from five months to 36 years with median age of 30 months [7]. It has a male predilection with male to female ratio of 2:1 [8]. Most of the patients are asymptomatic. However, patients can present with gross haematuria when the central lesions involve the renal pelvis [8]. Turner IR et al., as well as Yao DW et al., reported this tumour in 10-year-old male patients with abdominal pain as presenting complaint [5,6]. Piotrowski Z et al., presented a case of 21-year-old asymptomatic female [4]. In the present case, two-year-old male child presented with haematuria.
Metanephric adenofibroma is usually a solitary tumour, centered in the renal medulla in most cases [7]. Protrusion into pelvis has also been seen in a few cases [3]. However, to the best of our knowledge, till date no case has been reported with extension of tumour into urinary bladder. Tumours range in size from 1.8 cm to 11 cm with median size being 3.85 cm [7]. Turner IR et al., reported a 5 cm upper pole renal mass [5], Yao DW et al., described a 7 cm tumour in the middle of right kidney [6] while tumour size reported by Piotrowski Z et al., was 19 cm [4]. In the present case, the tumour measured 10 cm x 5.5 cm x 5 cm, occupying dilated pelvicalyceal system and protruding into pelvis and ureter. The mass protruded into the urinary bladder as a fleshy mass. Grossly, these tumours are usually solid but may be partially cystic. Cut surface is greywhite, yellow or tan [8]. Yao DW et al., described the tumour in their case report as firm, grey white with fibrous appearance [6]. The tumour in our case was also solid and greywhite.
Histologically, metanephric adenofibroma is an unencapsulated, sharply demarcated, composite tumour comprising of nodules of epithelium identical to metanephric adenoma embedded in sheets of spindle stromal cells [7-9]. The relative amounts of epithelial and stromal cell components vary from case to case. Shek TW et al., described a case with major stromal element and a minor epithelial element in the form of a small subcapsular nodule [2]. In our case, the stromal component was slightly more than the epithelial element. The epithelial component consists of small acini, tubules and papillary structures. Epithelial cells have small, uniform nuclei with delicate chromatin and inconspicuous nucleoli [9]. Cytoplasm is scant and pale or pink. Spindle cells are fibroblast- like with oval to fusiform nuclei and pale eosinophilic cytoplasm [9]. Onion skinning of stromal cells around epithelial components and angiodysplasia of intratumoural vessels may be seen [5,6]. Both these features were seen in our case. Mitotic figures are rare in both epithelial and stromal components. Psammoma bodies are common and may be numerous [8], as were seen in the present case. No blastemal component was found on extensive sampling in this case.
On immunohistochemistry, the epithelial component is typically positive for cytokeratin and WT-1 and negative for EMA [8,9]. The stromal component is frequently positive for CD34 [9]. Similar immunohistochemical profile was seen in our case except that we found the epithelial element to be positive for EMA. Also, focal CK7 positivity and Ki67 index <1% were seen in our case similar to what has been reported by Yao DW et al., [6]. Arroyo MR et al., have also described S100 and GFAP positivity in neuroglial nodules, if present [3]. No neuroglial nodules were seen in our case. However, presently there is no immunohistochemical profile to characterize metanephric adenofibroma.
Radiological features are nondiagnostic and indistinguishable from other paediatric solid renal tumours. Metanephric adenofibroma can potentially be mistaken as Wilms’ tumour [2]. It is distinguished from Wilms’ tumour by the smaller size of nuclei of epithelial cells and lack of blastema and mitotic activity [9]. The stromal component is benign and lacks the variety of differentiation that is often seen in Wilms’ tumour stroma. The stromal cells of Wilms’ tumour do not express CD34 [10]. It is important to distinguish between the two for management purpose. Metanephric adenofibroma is a benign tumour with no known recurrence or metastasis after surgical excision. On the other hand, Wilms’ tumour is a malignant tumour requiring chemotherapy. In the current case, the tumour involved the whole of right kidney, hence right nephroureterectomy was performed. No chemotherapy was given.
Conclusion
Metanephric adenofibroma is a rare benign renal tumour with only a few case reports. Rarely, it can present as a urinary bladder mass, clinically mimicking rhabdomyosarcoma and botryoid Wilms’ tumour. Awareness of such rare presentation of this entity is required for correct diagnosis and management in such cases.
[1]. Hennigar RA, Beckwith JB, Nephrogenic adenofibroma: A novel kidney tumour of young peopleAm J Surg Pathol 1992 16:325-34. [Google Scholar]
[2]. Shek TW, Luk IS, Peh WC, Chan KL, Chan GC, Metanephric adenofibroma: report of a case and review of the literatureAm J Surg Pathol 1999 23:727-33. [Google Scholar]
[3]. Arroyo MR, Green DM, Perlman EJ, Beckwith JB, Argani P, The spectrum of metanephric adenofibroma and related lesions: clinicopathologic study of 25 cases from the national wilms tumour study group pathology centerAm J Surg Pathol 2001 25:433-44. [Google Scholar]
[4]. Piotrowski Z, Canter DJ, Kutikov A, A-Saleem T, Pei J, Testa JR, Metanephric adenofibroma: robotic partial nephrectomy of a large’ tumour variantCan J Urol 2010 17:5309-12. [Google Scholar]
[5]. Turner IR, Tomaszewski JJ, Fox JA, Galambos C, Cannon GM Jr, Metanephric adenofibromaCan J Urol 2013 20:6737-38. [Google Scholar]
[6]. Yao DW, Qu F, Hu SW, Zheng JY, Wang JM, Zhu XY, Metanephric adenofibroma in a 10-year-old boy: report of a case and review of the literatureInt J Clin Exp Pathol 2015 8(3):3250-56. [Google Scholar]
[7]. MacLennan GT, Cheng L, Neoplasms of the kidney. In: Bostwick DG, Cheng L, editorsUrologic Surgical Pathology 2014 3rd edPhiladelphiaElsevier Saunders:127-128. [Google Scholar]
[8]. Tickoo SK, Reuter VE, Metanephric tumours other than Metanephric adenoma. In: Amin MB, Ticjoo SK, editorsDiagnostic Pathology Genitourinary 2016 2nd edSalt Lake City, UTElsevier:152-155. [Google Scholar]
[9]. Eble JN, sauter G, Epstein JI, Sesterhenn IA, World Health Organization Classification of Tumours. Pathology and genetics of tumours of the urinary system and male genital organs 2004 LyonIARC Press [Google Scholar]
[10]. Shao L, Hill DA, Perlman EJ, Expression of WT-1, Bcl-2 and CD34 by primary renal spindle cell tumours in childrenPaediatr Dev Pathol 2004 7(6):577-82. [Google Scholar]