Gastric cancer is the fifth most common cancer and the third most frequent cancer associated with death in the world [1]. In India, gastric carcinoma is the fifth most common cancer among males and seventh most frequent cancer among females [2].
The HER2 protein has been evaluated extensively and found amplified in 20% in breast cancers. The role of HER2 overexpression in gastric adenocarcinomas is estimated at 7-34% in gastric cancer and is related to poor survival rates [7]. With few reports from India, our aim was to study the same at a tertiary care center in Southern India [8-11]. We have also tried to study the association of HER2/neu and H. pylori with other clinicopathological parameters.
Materials and Methods
This cross-sectional study was conducted in a tertiary care hospital and medical college in Southern India. A total of 85 patients who had undergone subtotal or total gastrectomy for gastric cancer between January 2010 and September 2014 were included in the study. Mucosal biopsies were excluded. The proximal gastric cancers, which were included in esophageal or gastro-esophageal cancers, as per AJCC 7th edition, were excluded from our study [13]. Institutional Ethics Committee approval was sought. All cases with microscopic evidence of malignancy were included and all non-neoplastic lesions were excluded. The patients were separated into five groups: (i) <40 years; (ii) 41-50 years; (iii) 51-60 years; (iv) 61-70 years; and (v) >70 years. Clinical data, including age, sex, symptoms and metastatic sites were collected from the medical records. Three pathologists did careful screening of all the slides and a consensus opinion was recorded.
All specimens were fixed in formalin, analysed with routine Haematoxylin and Eosin (H&E) staining, HER2/neu status by IHC and H. pylori by Giemsa staining from uninvolved gastric tissue adjacent to tumour. HER2 IHC was done on formalin-fixed, paraffin-embedded tissue. Anti-HER 2/neu (4B5) Mouse Monoclonal Primary Antibody kit (Catalog no: AN753-5M, BioGenex Diagnostics, Hyderabad, India) was used following the manufacturer’s instructions. Antigen retrieval and unmasking was done by microwave irradiation for 10 minutes, three to four times. HER2/neu immunoreactivity was evaluated according to the previously defined scoring system proposed by Ruschoff et al., [Table/Fig-1] [14].
Grading system used for HER2/neu [14].
| HER2/neu IHC Score | HER2/neu IHC Pattern in Surgical Specimen | HER2/neu Expression Assessment |
|---|
| 0 | No reactivity or membranous reactivity in <10% of cancer cells | Negative by IHC |
| 1+ | Faint or barely perceptible membranous reactivity in ≥10% of cancer cells; cells are reactive only in part of their membrane | Negative by IHC |
| 2+ | Weak to moderate complete, basolateral or lateral membranous reactivity in >10% of tumour cells | Equivocal or weakly positive by IHC |
| 3+ | Strong complete, basolateral or lateral membranous reactivity in ≥10% of cancer cells | Positive |
All cases of 3+ scores were considered HER2/neu positive along with 2+ scores. The 2+ scores were also considered positive based on the observation that these patients also benefit from Trastuzumab therapy [14]. H. pylori colonization density using Giemsa (Merck, Mumbai, India) staining was graded from 0 to 3+ by a system proposed by Choudhary et al., [15].
Negative (Grade 0): H. pylori not detected;
1+ (Grade I): Occasional curved bacilli detected after an extensive search;
2+ (Grade II): H. pylori seen in fair number or in occasional clusters per 3 to 5 oil immersion fields in the mucus gland crypts or lumen in superficial submucosal lamina propria;
3+ (Grade III): Numerous bacilli in groups or in clusters present in almost oil immersion fields in the mucus layers.
Associations between HER2/neu and gender, tumour site, LVI, and histological subtype were analysed. Correlation of HER-2 status and H. pylori was also studied.
Statistical Analysis
Clinical and morphological characteristics were expressed as median and percentage. Chi-square test and Fisher’s exact test were used, to test the correlation between HER2 expression and the demographic (age, sex) and pathological (histological type, site, extent of local invasion, TNM staging, lymph node status, distant metastasis) variables. Statistical Package for Social Sciences (SPSS Inc, Chicago, IL, USA) Version 20.0, IBM was used to analyse the data. A p-value <0.05 was considered as statistically significant.
Results
A total of 85 gastrectomy specimens were included in the study. Age of the patients ranged from 22 to 84 years and mean age was 57.68±12.12 years, of which 67 (78.8%) were males and 18 (21.2%) were females. Out of the 85 cases studied, most cases 32% (27/85) were between 51 and 60 years while the least, 9% (8/85) <40 years. Location-wise, 78 of the 85 tumours were in the pyloric antrum and 7 in the body. All were adenocarcinomas. Fifty-five tumours (64.7%) were of the Lauren’s intestinal type and 35.3% (30) were diffuse. Mixed carcinomas were not seen within the study period. [Table/Fig-2] shows the clinical and pathological characteristics of patients included in the study.
Clinicopathological characteristics of the patients in the present study.
| Parameter | | Number (n=85) | % |
|---|
| Gender | Male | 67 | 78.8 |
| Female | 18 | 21.2 |
| Age range (Years) | | 22-84, 57.68+12.12 (Mean) | |
| Surgery type | Subtotal | 74 | 87.1 |
| Total | 11 | 12.9 |
| H. pylori | Positive | 59 | 69.4 |
| Negative | 26 | 30.6 |
| Tumour site | Antrum | 78 | 91.8 |
| Corpus | 7 | 8.2 |
| Histological type | Intestinal | 55 | 64.7 |
| Diffuse | 30 | 35.3 |
| Stage | I | 12 | 14.1 |
| II | 29 | 34.1 |
| III | 40 | 47.1 |
| IV | 4 | 4.7 |
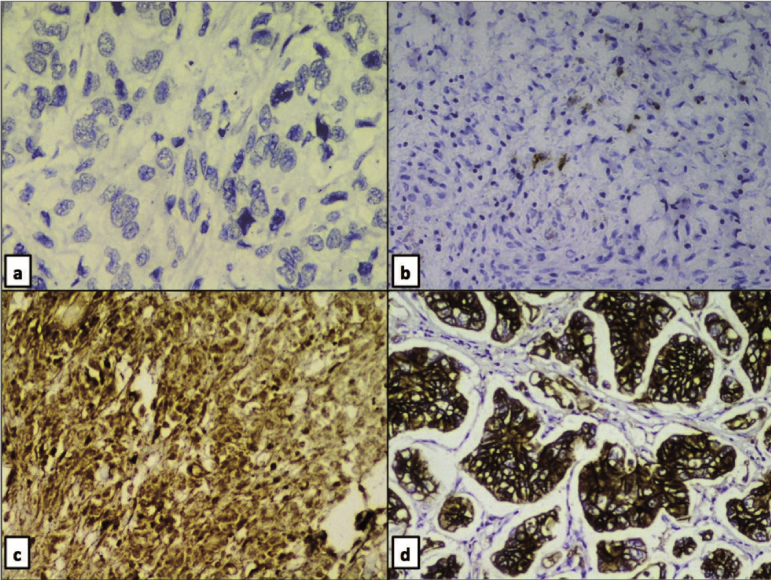
Most of the tumours 38.8% (33/85) were in T4a stage whereas, 9.4% (8/85) of the cases were in the early stage (T1b). Out of all the cases, 14.1% (12/85), 34.1% (29/85), 47.1% (40/85) and 4.7% (4/85) were in pathological TNM stage I, II, III and IV respectively. Out of the 85 cases, 64 cases (83.5%) were Score 0 and five cases (5.9%) were Score 1 for HER2/neu by IHC and hence are negative. Four cases (4.7%) were weakly positive (Score 2+) and twelve cases (14.1%) were strongly positive (Score 3+) [Table/Fig-3]. HER2/neu grade showed a significant correlation with intestinal type of carcinoma (14/16 HER2/neu positive cases, 87.5%, p=0.0429) and tumour TNM stage (16/16 cases >Stage II, 100%, p=0.018). However, HER2/neu status did not correlate significantly with tumour site (p=1.0).
HER2/neu immunoreactivity of cases: a) Negative - Grade 0; b) Negative - 1+; c) Weakly positive/equivocal - 2+; d) Strongly positive - 3+ (All: Immunohistochemistry, 40X).
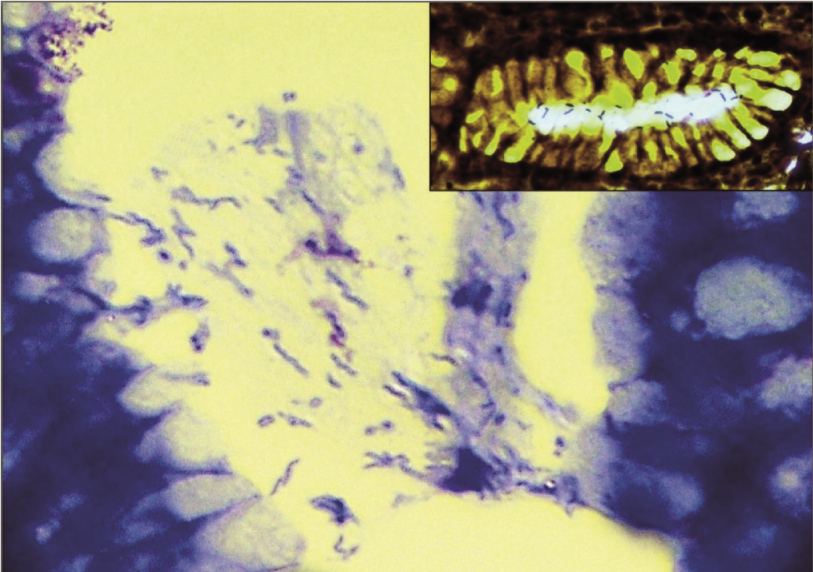
Adjacent mucosa showed severe H. pylori colonization only in 7.1% (6/85) cases, whereas it was moderate, mild and absent in 17.6% (15/85), 44.7% (38/85) and 30.6% (26/85) of cases respectively. Warthin Starry stain was done only in one case just for illustration purpose [Table/Fig-4]. Thirty eight of 55 (69.1%) intestinal type and 21/30 (70%) diffuse type adenocarcinomas demonstrated H. pylori by Giemsa stain in the adjacent mucosa. Severe colonization of H. pylori was seen in 3/30 (10%) of diffuse type, whereas it was 3/55 (5.5%) in intestinal type, which was statistically insignificant (p=0.73). H. pylori infection did not discriminate between intestinal (38/55, 69.1%) and diffuse (21/30, 70%) gastric cancer. Of the patterns of gastritis H. pylori caused, we found an associated intestinal metaplasia in 12 cases of intestinal gastric cancer and 4 cases of diffuse gastric cancer. According to H. pylori status, statistical significance was not present for gender, tumour location, and tumour histology (p=0.387, p=0.615, p=0.19, respectively). Relationship between HER2/neu and H. pylori is shown in [Table/Fig-5]. HER2/neu expression showed a negative correlation (r= -0.252) with density of H. pylori colonisation and was statistically significant using Spearman rank correlation (p-value = 0.020).
Moderate H. pylori colonization (Giemsa stain, 10X), Inset: Warthin-Starry stain (100X).
Association of pathological parameters and HER2/neu.
| Pathological parameters | HER2/neu Positive | HER2/neu negative |
|---|
| H. pylori | Positive | 10 | 59 |
| Negative | 6 | 20 |
| Histology | Intestinal | 14 | 41 |
| Diffuse | 2 | 28 |
| Stage | I | 0 | 12 |
| II | 2 | 27 |
| III | 12 | 28 |
| IV | 2 | 2 |
| Localization | Pyloric antrum | 15 | 63 |
| Corpus | 1 | 6 |
Discussion
Peptic ulcer disease, caused by pathogenic H. pylori, is a common problem in India. H. pylori has been detected in 95% of duodenal ulcers and 70%-80% of gastric ulcers [4]. H. pylori is also implicated in the etiology of gastric cancer. H. pylori and gastric cancer have been linked at varied rates (69%-97%) from several studies [16-18]. The eastern and southern areas of India show relatively increased prevalence of gastric cancer than Northern India [19]. Pandey A et al., observed that Indians are extremely prone to infection with carcinogenic H. pylori [20]. Nevertheless, due to the current sweeping abuse of antibiotics in the treatment of gastroenterological diseases, carcinogenic progression of H. pylori associated lesions has been stalled [21].
Research done on H. pylori infection in gastric cancer in different areas of India shows 50%-80% H. pylori positivity, concordant with 69.4% incidence detected in the present study [4,22]. Few studies done in India did not show any relation between H. pylori colonization and stomach cancer [23,24]. Kate V et al., studied 50 cases of gastric carcinoma along with 50 controls with non-ulcer dyspepsia. They found H. pylori infection less frequently in gastric carcinoma (38%) than those with non-ulcer dyspepsia (68%) [23]. Khanna AK et al., showed that 64.7% cases of gastric carcinoma and 74.4% cases of non-ulcer dyspepsia had colonization with H. pylori [24]. Meta-analysis by Huang JQ et al., in 2003 showed that H. pylori infection increases the risk of gastric cancer, both intestinal and diffuse types [25]. In our study as well, both intestinal and diffuse types of carcinoma showed equal incidence of H. pylori infection (intestinal, 38/55, 69.1%; diffuse, 21/30, 70%). Severe colonization was seen in 10% (3/30) of diffuse type whereas it was 5.5% (3/55) in intestinal type, an observation concordant with the results of Pereira et al., as well as Sipponen P et al., [26,27]. E-cadherin is a protein involved in cell to cell and cellular basal membrane adhesions. Loss of E-cadherin in tumours causes invasive progression of comparatively benign and rapid progression of tumours showing metastatic behaviour. Mutations in E-cadherin gene are seen mostly in poorly differentiated and diffuse type of cancers. Terres AM et al., found a highly significant association between H. pylori infection and E-cadherin protein loss (p<0.001), but the current study failed to reveal any significant association of dense H. pylori colonization with diffuse gastric cancer (p=0.73) [28].
Conversely, Araujo-Filho I et al., recorded a higher incidence of H. pylori infection in intestinal than diffuse type cancers [29]. Few other studies have also revealed significantly higher rates of H. pylori in intestinal type than the diffuse type of gastric cancer, the incidence ranging from as low as 27% to as high as 92% [30]. H. pylori-related gastritis has been proved to have a role in development of intestinal type of gastric cancer by indirectly inducing p53 mutations [31].
H. pylori colonization has been significantly related to intestinal metaplasia and dysplasia of precancerous lesions. Studies show that intestinal type of adenocarcinoma development is closely related to intestinal metaplasia [32]. In the present study, we found H. pylori associated intestinal metaplasia in 12 cases of intestinal gastric cancer. Intestinal metaplasia did not significantly correlate with intestinal type of cancer (Chi-square test, p=0.51), as per our study. A study with a bigger sample size over a longer period of time would have revealed a significant correlation. All atrophic gastritis cases showed intestinal type of cancer in association with strong H. pylori positivity. This finding, along with the high rate of occurrence of chronic gastritis in adjacent tissue, could be a predisposing condition for gastric carcinoma in our patient population.
Protein overexpression and high amplification of HER2/neu are seen in gastric carcinoma similar to breast carcinoma with few differences [33]. A 2+ score implying moderate/weak staining shows complete or basolateral membranous staining in ≥10% of the cells with a weak to medium intensity visible under medium magnification. A 3+ score implying strong staining implies complete or basolateral membranous staining in ≥10% of the neoplastic cells with very strong intensity visible under the lowest magnification. Both 3+ and 2+ scoring patients benefit from trastuzumab therapy [14]. Thus, 2+ score was considered positive in our study.
It should be pointed out that the present study was purely based on IHC and we have considered 2+ as weak positive. The 2+ results should be further be evaluated by Chromogenic In Situ Hybridisation (CISH)/ Fluoresecent In Situ Hybridisation (FISH) methods according to the current recommendations [14]. One of the drawbacks of our study is that the IHC 2+ cases were not confirmed by FISH analysis as per protocol due to economic constraints, and hence, our HER2/neu positivity rate may actually be lower. HER2/neu studies have shown excellent concordance rate (>95%) between IHC and FISH, in IHC 0 and IHC 3+ cases. Hence, these scores do not need FISH confirmation [34]. Bsng et al. reported that 26% of the score 2+ were FISH positive for HER2/neu overexpression, while in a study done by Shan L et al., 28.8% of equivocal were positive on FISH evaluation [35,36].
Many studies exhibit high HER2/neu overexpression in Gastro Esophageal Junction (GEJ) cancers compared to gastric cancers. HER2/neu overexpression may even be higher in esophageal cancers [35]. However, as per AJCC 7th edition 2012, we have included only cancers below 5 cm of GEJ in our study [13]. Hence, this could also be a reason for the lower frequency of HER2/neu expression in our study. All the tumours that showed HER2/neu positivity in our study were in the antropyloric area.
Our study revealed a significant relation between presence of HER2/neu and higher stages of carcinoma. This is in contrast to the findings of Tewari M et al., and Rajagopal I et al., who did not find any correlation between HER2/neu and tumour stage [9,11]. Interestingly, all cases in our study that showed a strong HER2/neu positivity were of the intestinal type. Only two cases of diffuse cancer showed 2+ positivity. None of the strongly HER2/neu positive cases belonged to a stage less than TNM tumour stage III. We can safely conclude that HER2/neu expression could lead to a higher stage of cancer and hence, have a worse prognosis.
We studied an area away from tumour in gastrectomies that is considered to be ideal to detect the presence or absence of H. pylori. Interestingly, our study demonstrated a negative statistical correlation between staining intensities for HER2/neu and H. pylori (p=0.02). This observation could not be compared with that of published literature due to absence of any such precedent finding. Tegtmeyer N et al., observed that H. pylori infection leads to activation of receptor tyrosine kinases like EGFR and HER2/neu. They describe that this activation leads to epithelial cell scattering and increased mitotic activity in these cells [37].
Limitation
Constraints of our study are dearth of adequate follow up and small sample size. Further large-scale studies are to be done in the future. Also, the HER2/neu 2+ cases, which are considered as equivocal, were not confirmed to be positive by FISH analysis due to financial constraints.
Conclusion
In contrast to other studies, absence of H. pylori infection in our study correlated with higher expression of HER2/neu. The implicit corollary that H. pylori infection protected the gastric cancer from expressing HER2/neu may be true in this part of the world where H. pylori infection is commonplace. However, this needs to be explored further by large cohort studies. All HER2/neu positive cases showed a higher stage of the intestinal variant of the gastric cancer. Thus, HER2/neu overexpression correlates with higher stage and leads to worse prognosis of the intestinal type of gastric cancer. HER2/neu expression is minimally present in the diffuse type of gastric cancer. H. pylori infection did not discriminate between intestinal and diffuse gastric cancer. All atrophic gastritis cases showed intestinal type of cancer in association with strong H. pylori positivity. It may be safely surmised that H. pylori infection that caused atrophic pattern of gastritis progressed to intestinal type of cancer. Thus, the pattern of gastritis in H. pylori infection should be studied and if an atrophic pattern is found, the gastroenterologist may be cautioned to this effect.