Correlation of Pregnancy Associated Plasma Protein A and Zinc with Calculated Risk Ratio of Dual Test
Indranil Ghoshal1, Bolar Suryakanth Varashree2, Vijetha Shenoy Belle3, Krishnananda Prabhu4
1 Consultant, Department of Biochemistry, Eastern Diagnostic India Limited, Kolkata, West Bengal, India.
2 Associate Professor, Department of Biochemistry, Kasturba Medical College(Manipal), Manipal Academy of Higher Education, Manipal, Karnataka, India.
3 Assistant Professor, Department of Biochemistry, Kasturba Medical College(Manipal), Manipal Academy of Higher Education, Manipal, Karnataka, India.
4 Professor, Department of Biochemistry, Kasturba Medical College(Manipal), Manipal Academy of Higher Education, Manipal, Karnataka, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Bolar Suryakanth Varashree, Associate Professor, Department of Biochemistry, Kasturba Medical College(Manipal), Manipal Academy of Higher Education, Manipal-576104, Karnataka, India.
E-mail: varashree.bs@manipal.edu
Introduction
First trimester screening by dual test is the most preferred method of antenatal screening. The detection rate of foetal aneuploidy using dual test is 95%. This test uses maternal serum free β human Chorionic Gonadotropin (free β hCG) and Pregnancy Associated Plasma Protein A (PAPP-A) along with maternal demographic and foetal sonographic indices to calculate risk for foetal aneuploidy. PAPP-A is a zinc binding metalloproteinase which is also responsible for placental development and foetal growth. So, zinc might also have some role in first trimester screening. No studies are available substantiating the role of zinc in first trimester screening.
Aim
To measure and correlate maternal serum zinc with PAPP-A and calculated risk ratio of first trimester screening.
Materials and Methods
PAPP-A and zinc were measured from the serum of 84 pregnant women aged 20-40 years in 11th-13th weeks+6 days of gestation who underwent dual test during their antenatal check-up. Risk calculation was done using Screening Software for Downs Windows Lab (SSDWL).
Results
Maternal serum PAPP-A showed a significant positive correlation with maternal serum zinc (p-value <0.001). Maternal serum PAPP-A and zinc both showed a significant positive correlation with calculated risk ratio (p-value<0.01).
Conclusion
As PAPP-A is a zinc binding metalloproteinase, zinc showed significant pattern of correlation with aneuploidy risk as shown by PAPP-A. So there could be a possible role for serum zinc in first trimester of screening.
First trimester screening, Metalloproteinases, Trisomy
Introduction
First trimester screening for aneuploidy has gained more relevance and importance due to increasing number of couples conceiving at higher age [1]. This has created an increased demand for different antenatal screening tests for chromosomal aneuploidies. Initially for this purpose Nuchal Translucency (NT), the sonographic appearance of subcutaneous accumulation of fluid behind the neck of foetus in the first trimester, along with maternal age was used for screening Down syndrome with 75% success rate [2,3]. Later, it was realized that when maternal free β hCG and PAPP-A were also taken into account for this screening, rate of detection of Down syndrome improved to about 85%-90% [3,4]. When absence of the nasal bone (a sonography finding on the foetus indicating trisomy 21) along with the above biochemical markers and maternal demographic characteristics were used, the detection rate of foetal aneuploidy in first trimester rose to more than 95% [2-5]. Hence this is one of the most commonly used aneuploidy screening methods and is called as dual test.
PAPP-A produced by the syncytiotrophoblast of the placenta is a zinc binding metalloproteinase belonging to the metzincin superfamily [6-8]. PAPP-A has an elongated zinc-binding motif, with residues coordinating the catalytic zinc ion of the active site, and a structurally important methionine residue called Met-turn, both of which are strongly conserved within the metzincin superfamily of metalloproteinases [9]. In all catalytic sites of such proteins, the zinc ion plays important role in catalysis by behaving as a Lewis acid. Zinc ion also stabilizes the tertiary structure of the enzymes and removal of the bound zinc can lead to a loss of enzymatic activity in such proteins [10].
The concentration of PAPP-A in maternal circulation increases very rapidly in first trimester with a doubling time of three to four days [8]. Decreased maternal serum PAPP-A level has been linked to abnormal placental function [11]. However, dual test does not take into account serum zinc. There have been no studies correlating serum zinc with PAPP-A and aneuploidy risk. So, this study attempted to correlate serum zinc, PAPP-A and aneuploidy risk ratio generated by dual test in pregnant women aged 20-40 years in first trimester of pregnancy.
Materials and Methods
This cross-sectional study was carried out in Department of Biochemistry, Kasturba Medical College, Manipal, Karnataka, India, from December 2013 to March 2015, after obtaining approval from the Institutional Ethical Committee (IEC No 437/2013). Informed consent was obtained from study subjects prior to the blood collection. In order to estimate a correlation coefficient of 0.3 to 5% level of significance for power of 80% adjusting for age stratification into two categories, minimum of 84 sample size was required. Left over samples of 84 pregnant women aged 20-40 years in 11th-13th weeks +6 days of gestation who underwent dual test during their first trimester antenatal check-up were used for measuring serum zinc levels. Pregnant women before 11th week and after 14th week of gestation, with previous history of gestational diabetes, with disorders requiring long term medications and women with twin pregnancy were excluded from this study.
Study subjects were divided based on maternal age into Group 1 (21-30 years) and Group 2 (31-40 years)
Biochemical analysis: Serum PAPP-A was measured by Electrochemiluminescence Immunoassay (ECLIA) (Roche diagnostics).
Serum zinc was measured by colorimetric method using a specific kit made by Crest Biosystems [12].
Risk calculation: Risk calculation was done using Screening Software for Downs Windows Lab (SSDWL) made by SBP software and recommended by American College of Obstetricians and Gynaecologists (ACOG) [13]. As sonographic and biochemical markers are bound to change with gestational age which might influence the outcome of these tests, we also compared PAPP-A across different weeks of gestations [14]. The software was used to screen the pregnant women for trisomies. The demographic details of the patient, age, Body Mass Index (BMI), NT, Crown Rump Length (CRL), weeks of gestation, serum free β HCG and PAPPA of the patient when entered into the software will give the risk ratio for trisomies.
Statistical Analysis
Independent t-test, Pearson’s correlation, Spearman’s correlation, and ANOVA was used appropriately for statistical analysis.
Results
In the present study maximum of the study subjects belonged to Group 1 (21-30 years) [Table/Fig-1].
Age distribution of the study subjects.
| Age group | Age (years)Mean± SD | Number | Percentage |
|---|
| Group 1(21-30 years) | 26.64 ± 2.33 | 59 | 70.23% |
| Group 2(31-40 years) | 33.72 ± 2.82 | 25 | 29.77% |
SD - Standard deviation
Only 13 study subjects belonged to Group A (11th week of gestation) [Table/Fig-2]. The correlation between the zinc and Multiple of median (MoM) of PAPP-A with different weeks of gestation was found to be insignificant. (p-value 0.164 and 0.332) [Table/Fig-3]. Similar results for age group were observed (p-value was non-significant) [Table/Fig-4].
Comparison of age of subjects based on weeks of gestation.
| Week of gestation | Number | Percentage | Age (years)Mean± SD | *p-value |
|---|
| Group A(11th week) | 13 | 15.47% | 30.23 ± 5.46 | 0.283 NS |
| Group B(12th week) | 52 | 61.9% | 28.69 ± 3.78 |
| Group C(13th week) | 19 | 22.61% | 27.89 ± 3.78 |
*ANOVA; NS = Not significant
Comparison of serum zinc and MoM of serum PAPP-A levels among subjects in different weeks of gestation.
| Parameters | Group A (n=13)(11th week) | Group B (n=52)(12th week) | Group C (n=19)(13th week) | p-value |
|---|
| Zinc (μg/dL)Mean±SD | 72.53 ± 14.62 | 68.09 ± 13.15 | 75.05 ± 16.60 | *0.164 NS |
| MoM of PAPP-A(mIU/L)Median and IQR | 0.97(0.62,1.72) | 0.97(0.63,1.34) | 1.16(0.62,1.72) | #0.332 NS |
*ANOVA, #Mann Whitney U test, MoM = Multiple of median, IQR = Inter quartile range, NS = Not significant
Comparison of serum zinc and MoM of serum PAPP-A levels among subjects in different age groups.
| Parameters | Group 1 (n = 59)(21-30 years) | Group 2 (n =25)(31-40 years) | p-value |
|---|
| Zinc (μg/dL)Mean ± SD | 69.48 ±13.54 | 70.72 ± 14.78 | *0.718 NS |
| MoM of PAPP-A (mIU/L)Median and IQR | 0.98 (0.62,1.49) | 1.10 (0.65,1.52) | #0.765 NS |
*Independent t-test
#Mann-Whitney U test
NS = Not significant
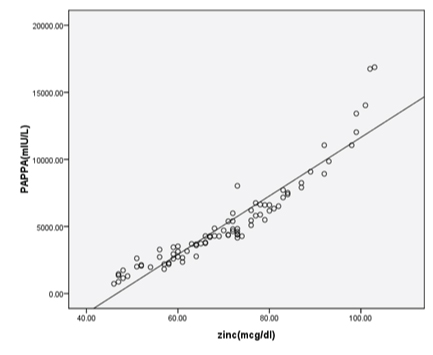
Significant positive correlation between maternal serum zinc and PAPP-A was observed supporting zinc binding nature of PAPP-A [Table/Fig-5].
Correlation between maternal serum zinc with PAPP-A (r = 0.972, p<0.001).
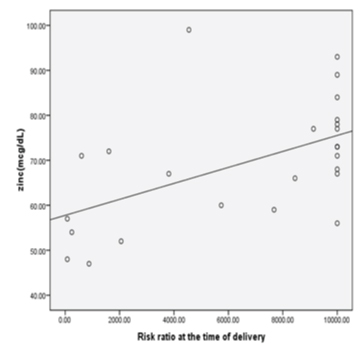
Significant positive correlation between maternal serum zinc and aneuploidy risk ratio in Group 2 subjects was observed indicating low levels of zinc can predispose to high risk of foetal aneuploidy among subjects in higher age group [Table/Fig-6].
Correlation between maternal serum zinc with calculated aneuploidy risk ratio in Group 2 (r=0.598, p=0.002).
Maternal serum PAPP-A showed a significant positive correlation with calculated risk ratio in subjects with higher age group [Table/Fig-7].
Correlation of PAPP-A with calculated aneuploidy risk ratio at the time of delivery.
| Parameter | r value | *p-value |
|---|
| Risk ratio at the time of delivery | 0.30 | 0.01 |
| Risk ratio at the time of delivery in Group 1 (n = 59) | 0.190 | 0.15 NS |
| Risk ratio at the time of delivery in Group 2 (n = 25) | 0.591 | 0.002 |
*Spearman correlation
NS = Not significant
Discussion
Down syndrome and zinc deficiency are not a common occurrence due to increased health awareness and availability of screening methods. PAPP-A, a zinc containing protein was already an established parameter included in aneuploidy screening [15,16]. This study attempted to associate low zinc levels with respective aneuploidy risk scores and PAPP-A. As this study showed a strong correlation between serum zinc and calculated aneuploidy risk scores and PAPP-A, it has a potential to be included to the existing parameters used in aneuploidy risk calculation algorithms.
Even though chorionic villus sampling is the definitive test, it cannot be used for routine screening due to its inherent risk and cost. So, non-invasive biochemical tests using algorithm became popular for screening of such cases. Previous studies have observed sensitivity and validity of test increases by including more number of parameters for aneuploidy screening [4,5]. So including serum zinc to the risk calculation algorithm may further improve its sensitivity.
As literature states that, maternal age is an important predictor used for foetal aneuploidy screening and can significantly influence these biochemical parameters, this study categorised the subjects in two groups to evaluate possible impact of maternal age in risk calculation with respect to zinc and PAPP-A levels [Table/Fig-1] and majority of patients underwent screening test in the 12th week of gestation [Table/Fig-2]. Age and gestational related cut-off values of zinc and PAPP-A could not be determined to comment on decreased or increased levels as PAPP-A shows wide fluctuations among normal population due to which it has been expressed in MoM. So in such cases no definite cut-off can be established and algorithms have been developed only by associating population MoM with other parameters for aneuploidy risk calculations. There was no significant difference in serum zinc and MoM of serum PAPP-A levels between these groups [Table/Fig-4].
Maternal serum zinc showed a significant positive correlation with PAPP-A supporting the association between these two parameters [Table/Fig-5]. The role of zinc was further substantiated by our finding that low levels of maternal serum zinc strongly correlated with high aneuploidy risk for the baby among higher age group pregnant women [Table/Fig-6]. Maternal serum PAPP-A showed a significant positive correlation with calculated risk ratio in subjects with higher age group [Table/Fig-7]. This study could only associate low zinc levels with calculated risk scores to evaluate a possibility of this to be included in the risk calculation algorithm. The possible role of zinc and zinc deficiency in relation to Down syndrome need to be further explored.
Limitation
In the present study, none of the cases had Down syndrome or zinc deficiency. As artificial zinc deficiency model could not be created in humans due to ethical reasons, we could only associate low zinc levels with aneuploidy risk score and PAPP-A. The sample size in this study was also small which might have influenced our results.
Conclusion
As low levels of maternal serum zinc strongly correlated with PAPP-A and aneuploidy risk, it has a potential to be included in antenatal screening to further improve its specificity and sensitivity. Similar study in a larger number of patients can help us to ascertain the inclusion of zinc to the existing risk calculation algorithm for better and earlier detection of foetal aneuploidy.
SD - Standard deviation
*ANOVA; NS = Not significant
*ANOVA, #Mann Whitney U test, MoM = Multiple of median, IQR = Inter quartile range, NS = Not significant
*Independent t-test
#Mann-Whitney U test
NS = Not significant
*Spearman correlation
NS = Not significant
[1]. Dunson DB, Baird D, Colombo B, Increased infertility with age in men and womenObstet Gynecol 2004 103(l):51-56. [Google Scholar]
[2]. Dutta DC, Antenatal care, preconceptional counseling and care. In: Konar H, editorDC Dutta’s textbook of Obstetrics 2015 8th editionIndiaJaypee Brothers Medical Publishers (P) Ltd:106-18. [Google Scholar]
[3]. Cicero S, Sonek JD, McKenna DS, Croom CS, Johnson L, Nicolaides KH, Nasal bone hypoplasia in trisomy 21 at 15–22 weeks’ gestationUltrasound Obstet Gynecol 2003 21:15-18. [Google Scholar]
[4]. Cicero S, Rembouskos G, Vandecruys H, Hogg M, Nicolaides KH, Likelihood ratio for trisomy 21 in fetuses with absent nasal bone at the 11–14 weeks scanUltrasound Obstet Gynecol 2004 23:218-23. [Google Scholar]
[5]. Cicero S, Longo D, Rembouskos G, Sacchini C, Nicolaides KH, Absent nasal bone at 11–14 weeks of gestation and chromosomal defectsUltrasound Obstet Gynecol 2003 22:31-35. [Google Scholar]
[6]. Viorica Radoi LC, Bohiltea. Pregnancy-associated plasma protein A and pregnancy outcomesMaternal Fetal Medicine 2009 5(1):16-20. [Google Scholar]
[7]. Conover CA, Key questions and answers about pregnancy-associated plasma protein-ATrends Endocrinol Metab 2012 23(5):242-49. [Google Scholar]
[8]. Bischof P, DuBerg S, Herrmann W, Sizonenko PC, Pregnancy associated plasma protein A and hCG in early pregnancyBr J obstet Gynaecol 1981 88(10):973-75. [Google Scholar]
[9]. Conover CA, Bale LK, Overgaard MT, Johnstone EW, Laursen UH, Füchtbauer EM, Metalloproteinase pregnancy-associated plasma protein A is a critical growth regulatory factor during fetal developmentDevelopment 2004 131(5):1187-94. [Google Scholar]
[10]. McCall KA, Huang C, Fierke CA, Function and mechanism of zinc metalloenzymesJ Nutr 2000 130(5):1437S-46S. [Google Scholar]
[11]. Westergaard JG, Teisner B, Grudzinskas JG, Serum PAPP-A in normal pregnancy: relationship to fetal and maternal characteristicsArch Gynecol 1983 233(3):211-15. [Google Scholar]
[12]. Abe A, Yiamashita S, Colorimetric method for the estimation of zincClin Chem 1989 35(4):552-54. [Google Scholar]
[13]. Wøjdemann KR, Shalmi AC, Christiansen M, Larsen SO, Sundberg K, Brocks V, Improved first-trimester Down syndrome screening performance by lowering the false-positive rate: a prospective study of 9941 low-risk womenUltrasound Obstet Gynecol 2005 25:227-33. [Google Scholar]
[14]. Picklesimer AH, Moise KJ, Wolfe HM, The impact of gestational age on the sonographic detection of aneuploidyAm J Obstet Gynecol 2005 193(3.2):1243-47. [Google Scholar]
[15]. Shiefa S, Amargandhi M, Bhupendra J, Moulali S, Kristine T, First trimester maternal serum screening using biochemical markers PAPP-a and free β-hcg for down syndrome, patau syndrome and edward syndromeIndian Journal of Clinical Biochemistry 2013 28(1):03-12. [Google Scholar]
[16]. Leguy MC, Brun S, Pidoux G, Salhi H, Choiset A, Menet MC, Pattern of secretion of pregnancy-associated plasma protein-A (PAPP-A) during pregnancies complicated by fetal aneuploidy, in vivo and in vitroReproductive Biology and Endocrinology 2014 12:129 [Google Scholar]