HNC is the sixth most common type of cancer in the world, representing about 6% of all cancer cases [1]. Oral cancer is the most common type of cancer in India amongst men, and the third most frequently occurring cancer in India amongst both men and women [2]. Overall, 57.5% of global HNC occur in Asia, especially in India with over 200,000 new cases and over 100,000 deaths occurring each year [3]. Patients with HNC are usually treated with surgery, Radiotherapy (RT), Chemotherapy (CT), or a combination of both of these.
Conventional Fractionation (CF) RT is the standard approach to treat the patients with HNC, however it may not be the optimal treatment owing to the failures caused by the proliferation of clonogen cells [4]. In an attempt to improve the local control and Overall Survival (OS) rates, researchers have tried the use of altered treatment regimens, including Hyper Fractionated (HF) RT and accelerated RT.
A major cause of treatment failure in HNC is the proliferation of clonogen cells [5]. The technique of hyperfractionation is implied to overcome this limitation keeping in mind the innate radiobiology of the tumour cells and subsequent alteration of RT schedule. However, severe acute reactions have curtailed the feasibility of the same [5]. Concomitant Boost RT (CBT) is a variant of accelerated fractionation and is associated with minimal enhancement of acute reactions while minimising the volume of tissue that is irradiated with high doses. The CBT was designed to shorten overall length of treatment thereby diminishing the opportunity for accelerated repopulation of clonogenic cells during therapy [5,6]. Since, it significantly reduces the total treatment time, it is of particular relevance in high volume centers [7].
RT and CT-RT in HNC patients, especially with two dimensional techniques on Cobalt-60 are prominently associated with chief toxicities of mucositis and dermatitis in face and neck region. Clinically, these lead to the complaints of pain, odynophagia as well as increase the risk of infections, ulcers and wounds. This in turn limits oral intake leading to malnutrition and a negative impact on the diet and overall quality of life of patients [8].
The department that conducted the study caters to a large volume of patients with HNC. We intended to formulate a treatment regimen that reduces the overall treatment time with comparable toxicity profile and outcomes as the conventional regimen. This would entail the possibility of treating more number of patients and reducing the waiting time. Hence, the present study was carried out to compare toxicities (mucositis, skin reactions, dysphagia and xerostomia) of these two RT schedules for oral and oropharyngeal cancers.
Materials and Methods
This prospective study was carried out at the Department of Radiation Oncology, Gandhi Medical College, Bhopal between July 2015–June 2016. A total of 60 patients at our institute were assigned in this study. Patient accrual was done after applying inclusion and exclusion criteria in a 1:1 manner. Prior approval was taken from the Institutional Ethics Board. Patients aged <70 years (both sexes) with histologically proven locally advanced oral cavity/oropharyngeal Squamous Cell Carcinomas (SCC), with Karnofsky Performance Score ≥70, normal baseline haematological investigations and chest X-ray were included. Patients aged >70 years, with distant metastasis, prior head and neck irradiation/surgery or histology other than SCC were excluded from the study.
Patients were assessed and staged clinically in majority of cases. Chest X-ray and ultrasound of the abdomen was done as a routine work up. Computerized tomography scan was desirable for precise loco regional disease assessment but could not be done uniformly owing to logistic issues. All the patients received induction CT with three cycles of injection paclitaxel (175 mg/m2) and injection cisplatin (75 mg/m2), repeated every three weeks. This was followed by allocation into one of the two study arms.
A total of 60 patients (30 in each arm) were included in the study. Patients were treated on cobalt-60 theratron 780 machine. A total dose of 7000 centigray (cGy)/35 fraction @ 200 cGy/ fraction /day, five days a week over seven weeks were prescribed in Group A (CF arm); and a dose of 6900cGy @ 180 cGy/ fraction five days a week for six weeks, and 150 cGy (as a boost dose field-in-field) in the last 10 fractions with an inter fraction gap of 4-6 hours was prescribed in Group B (CBT arm). Toxicities were analysed weekly, and one and three months after treatment completion as per the radiation therapy oncology group acute radiation morbidity scoring system [9]. Additionally, group B toxicity assessment was continued one week after completion of the treatment to correspond with group A treatment duration. Separate assessment of mucosal reactions, skin reactions, dysphagia and xerostomia was made. Statistical analysis was done by the online graph pad software (©2017 GraphPad Software, Inc. 7825 Fay Avenue, Suite 230 La Jolla, CA 92037 USA) using Chi-square test. A value of p<0.05 was considered significant.
Results
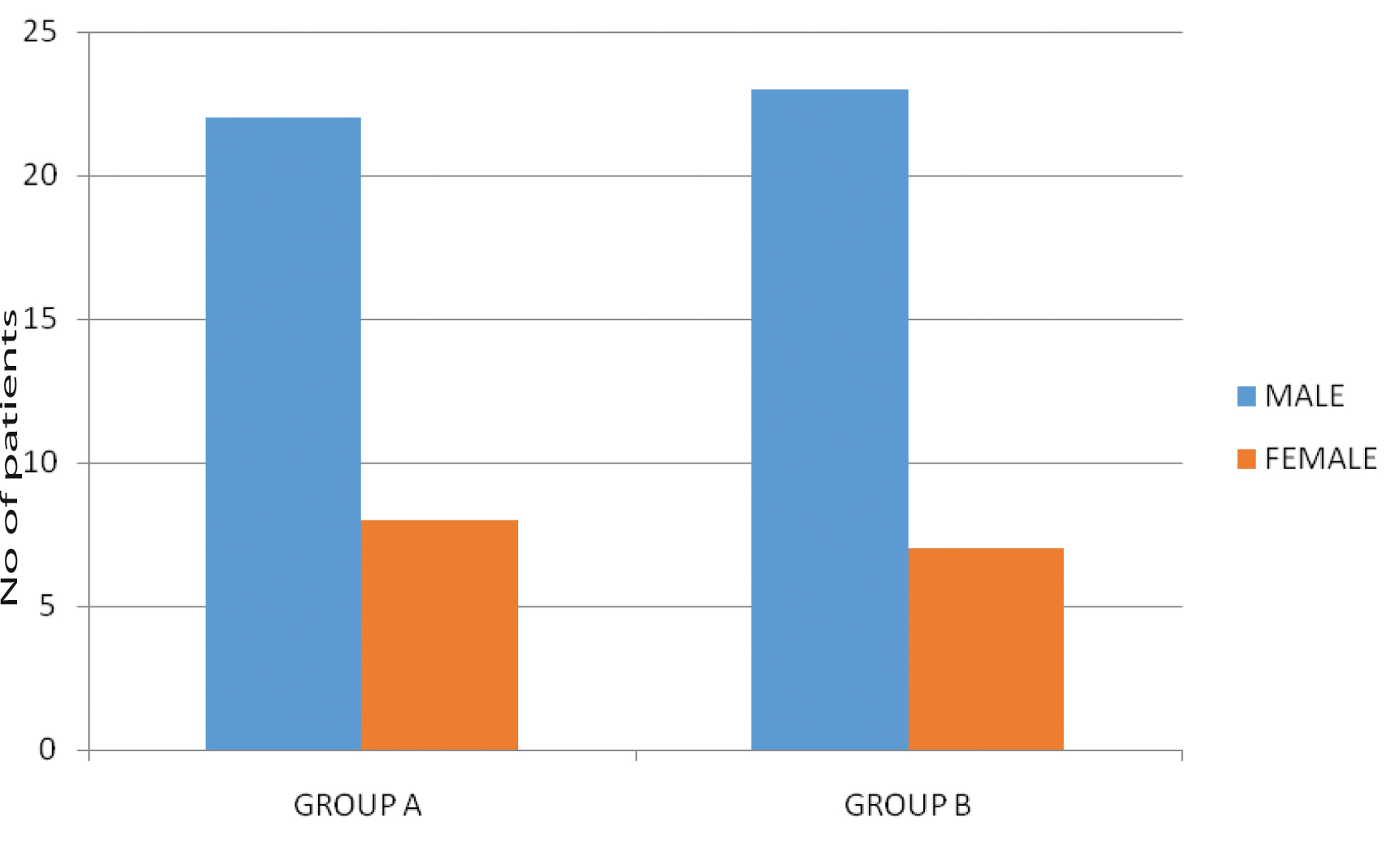
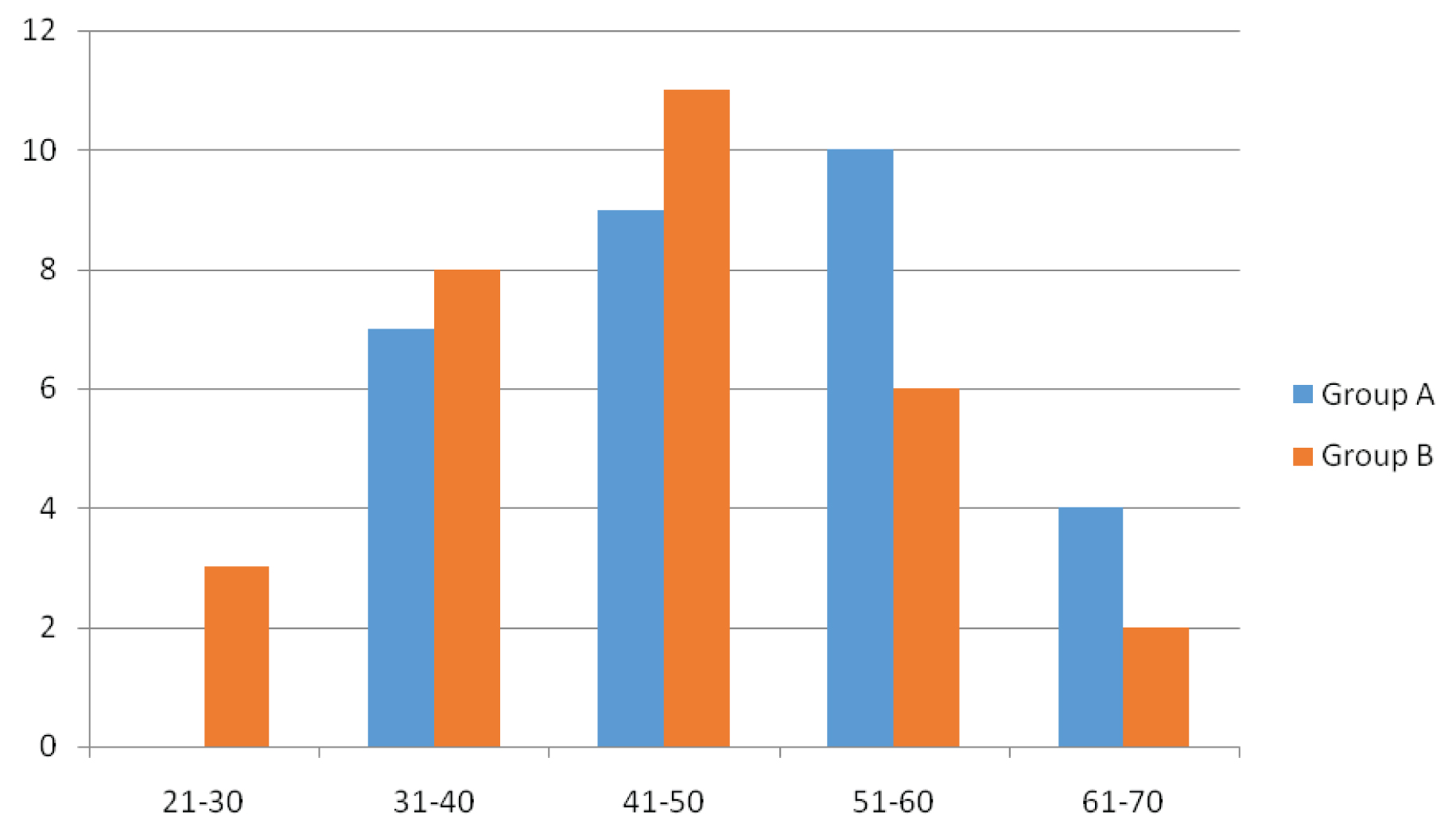
Total 60 patients were enrolled in the study; overall mean age was 47.35 years (range 23-70 years). Mean age for Group A was 50.33 years (range 32-70 years) and for Group B was 44.36 years (range 23-70 years). In the present study, maximum patients belonged to 4th- 6th decade of life. The patient and disease characteristics has been shown in [Table/Fig-1,2,3 and 4]. In this study, 22/30 (73.33%) patients in Group A, and 23/30 patients (76.66%) in Group B were males while 8/30 patients (26.66%) in Group A and 7/30 patients (23.33%) in Group B were females. Age distribution showed 10/30 patients (33.33%) in Group A belonged to age group 51-60 years. while 11/ 30 (36.66%) patients in Group B belonged to age group 41-50 years. Carcinoma tongue (33.33%) was the most common subsite in Group A (10/30) while carcinoma buccal mucosa (50%) was the most common in Group B (15/30). All the patients belonged to stage IVa. The TNM distribution is as shown in [Table/Fig-4]. Commonest presenting symptoms among the patients were ulceration, ulceroprolifrative growth, pain, discharge, trismus and difficulty in swallowing.
| S. No. | Site | Group A | Group B |
|---|
| No. of Patients (%) | No. of Patients (%) |
|---|
| 1 | Alveolus | 4(13.33%) | 5(16.66%) |
| 2 | Buccal Mucosa | 8(26.66%) | 15(50%) |
| 3 | Tongue | 10(33.33%) | 7(23.33%) |
| 4 | Floor of Mouth | 2(6.66%) | 0(0) |
| 5 | Base of Tongue | 1(3.33%) | 2(6.66%) |
| 6 | Lips | 1(3.33%) | 0(0) |
| 7 | Tonsillar Fossa | 3(10%) | 0(0) |
| 8 | Hard Palate | 1(3.33%) | 1(3.33%) |
TNM distribution in Groups A and B.
The overall appearance of the toxicities assessed is summarised in [Table/Fig-5].
Overall toxicity in both arms.
| Toxicity | Group A | Group B |
|---|
| Number of Patients | % | Number of Patients | % |
|---|
| Mucosal |
| Grade I | 18 | 60% | 18 | 60% |
| Grade II | 12 | 40% | 10 | 33.33% |
| Grade III | 0 | 0 | 2 | 6.66% |
| Skin |
| Grade I | 27 | 90% | 25 | 83.33% |
| Grade II | 3 | 10% | 5 | 16.66% |
| Dysphagia |
| Grade I | 18 | 60% | 18 | 60% |
| Grade II | 12 | 40% | 12 | 40% |
| Xerostomia |
| Grade I | 27 | 90% | 21 | 70% |
| Grade II | 3 | 10% | 9 | 30% |
Mucosal reactions: Grade I reaction was seen in18 (60%) patients, Grade II reaction in 12 (40%) patients in Group A while in Group B, 18 (60%) patients showed Grade I, 10 (33.3%) patients showed Grade II reactions while two patients (6.66%) showed Grade III reactions. Weekly and post treatment assessment (Group A and B) is as shown in [Table/Fig-6].
Weekly and post treatment assessment of mucosal toxicity in Group A and B.
| Group A | Group B |
|---|
| Grades | During RT Weekly | Post RT (1 and 3 Months) | During RT Weekly | Post RT (1 and 3 Months) |
|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 1 | 3 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 1 | 3 |
|---|
| I | - | 620% | 1446.6% | 1653.3% | 1860% | 1033.3% | 1240% | 826.66% | - | - | 413.3% | 826.6% | 1550% | 1860% | 1550% | 1033.3% | - | - |
| II | - | - | 1033.3% | 26.66% | 1240% | 1033.3% | 1030.3% | - | - | - | - | 26.6% | 1033.3% | 826.6% | 826.6% | 516.6% | - | - |
| III | - | - | - | - | - | - | - | - | - | - | - | - | - | 26.6% | - | - | - | - |
| IV | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
Skin reactions: Grade I reaction were seen in 27 (90%) patients, Grade II reaction in-3 (10%), and no Grade III reactions were seen in group A while in group B, 25 (83.3%) patients showed Grade I and 5 (16.6%) patients showed Grade II reactions. Weekly and post treatment assessment (Group A and B) is as shown in [Table/Fig-7].
Weekly and post treatment assessment of skin toxicity in Group A and B.
| Group A | Group B |
|---|
| Grades | During RT Weekly | Post RT (1 and 3 Months) | During RT Weekly | Post RT (1 and 3 Months) |
|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 1 | 3 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 1 | 3 |
|---|
| I | - | 2066.6% | 2170% | 2790% | 2790% | 2273.3% | 2066.6% | 516.6% | - | - | - | 2273.3% | 2583.3% | 2376.6% | 2376.6% | 2273.3% | 1033.3% | - |
| II | - | - | - | 310% | 310% | 26.6% | 26.6% | - | - | - | - | - | 310% | 516.6% | 516.6% | 413.3% | 26.6% | - |
| III | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| IV | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
Dysphagia: Grade I toxicity was similar in both groups and seen in 18 patients out of 30 (60%) each, while 12 patients out of 30 (40%) patients in Group A and12 patients out of 30 (40%) patients in Group B had Grade II toxicity. Weekly and post treatment assessment (Group A and B) is as shown in [Table/Fig-8].
Weekly and post treatment assessment of dysphagia in Group A and B.
| Group A | Group B |
|---|
| Grades | During RT Weekly | Post RT (1 and 3 Months) | During RT Weekly | Post RT (1 and 3 Months) |
|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 1 | 3 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 1 | 3 |
|---|
| I | - | 723.3% | 1343.3% | 1653.3% | 1860% | 1033.3% | 1240% | 826.6% | - | - | 413.3% | 826.6% | 1550% | 1860% | 1550% | 1033.3% | 413.3% | - |
| II | - | - | 1136.6% | 26.66% | 1240% | 1033.33% | 1033.33% | - | - | - | - | 26.6% | 1240% | 1033.3% | 826.6% | 516.6% | - | - |
| III | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| IV | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
Xerostomia: The incidence of xerostomia after radiotherapy in the patients in both Groups indicates that 27 patients out of 30 in Group A (90%) and in Group B 21 patients out of 30 (70%) patients had Grade I toxicity and three patients out of 30 (10%) patients in Group A and nine patients out of 30 (30%) patients in Group B had Grade II toxicity. Weekly and post treatment assessment (Group A and B) is shown in [Table/Fig-9].
Weekly and post treatment assessment of xerostomia in Group A and B.
| Group A | Group B |
|---|
| Grades | During RT Weekly | Post RT (1 and 3 Months) | During RT Weekly | Post RT (1 and 3 Months) |
|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 1 | 3 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 1 | 3 |
|---|
| I | - | - | 516.6% | 1550% | 2790% | 2790% | 2790% | 2790% | - | - | - | - | 1860% | 2170% | 2170% | 2170% | 2170% | - |
| II | - | - | - | 310% | 310% | 310% | 310% | 310% | - | - | - | - | 620% | 723.3% | 723.3% | 930% | 620% | - |
| III | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| IV | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
On statistical analysis using Chi-square test, no significant difference in toxicity was found between the two arms in terms of mucositis (p=1), skin reactions (p=0.6404), dysphagia (p=0.7906) and xerostomia (p=0.1066).
Discussion
Carcinoma of oral cavity and oropharynx is the commonest malignancy seen among the Indian population [3]. The incidence of mucositis is reported to be between 85%-100% depending on the treatment regimen (CFRT/altered fractionated RT/CT-RT) while the incidence of skin reactions is postulated to be 7-25% varying between different types of cancer treatment regimens [10]. We also assessed the appearance and severity of dysphagia and mucositis. Usually the toxicities tend to be on the higher side while treating patients with Cobalt-60.
In oropharyngeal cancers, a ten day reduction in overall treatment time to around five weeks is estimated to yield a 10-15% improvement in local control [7,11]. Most of the patients in the present study were suffering from carcinoma of tongue (Group A=33.3%) and buccal mucosa (Group B= 50%). This may be attributed to the excessive tobacco use and poor oral hygiene prevalent in the population catered.
Mucositis: A well-known side effect of RT is mucositis [11] and has been defined as the inflammatory change of the oral mucosa as a direct effect of RT [12]. Beumer J et al., postulated that inhibition of cell growth and maturation by radiation disrupts the primary mucosal barrier of the mouth and throat [13]. This further leads to oropharyngeal infection by resident oral microflora. This is clinically manifested as oral mucositis, oral candidiasis, xerostomia, trismus, dental caries, osteoradionecrosis, cellulitis, and viral mucosal eruptions [14]. These complications cause significant discomfort to the patient and are responsible for delays or dosage limitation in cancer treatments. In addition, severe mucositis often requires temporary or permanent cessation of RT before planned completion. This is undesirable in the treatment of HNC as prolongation of overall treatment time has been shown to adversely affect the radiocurability of HNC [15]. In our study, the appearance Grade III mucosal toxicity was reported only in two patients (6.66%) of Group B. This finding differs from the study of Vees H et al., and De Arruda FF et al., who detected Grade I to II mucositis [16,17]. In addition, the considerably less Grade III mucosal toxicity documented in our results is in contrast with other studies quoted in literature [18,19]. A possible reason could be that we paid a high emphasis towards nutritional counselling, generous use of non steroidal anti-inflammatory drugs (preferential cyclo-oxygenase-2 inhibitors), oral hygiene and use of benzydamide hydrochloride 0.15% and chlorhexidine solution as mouthwash. Literature reveals studies reporting a similar effect with these measures [20,21]. In our study, the maximum incidence of mucositis was observed in fifth and sixth week of treatment that was consistent with observation of Medina JA et al., [19] and Majdeen M et al., [22].
Skin reaction: Skin reaction is another important toxicity encountered with RT. Our study showed the incidence of skin reactions in Group A to be Grade I in 66% of the patients by the end of second week, and increased to affect 90% patients by the end of fourth and fifth weeks. In addition Grade II reactions were also seen in 10% patients at the end of fourth week of treatment. By the end of the seventh week, Grade I reactions were seen in 66.6% patients while 6.6% patients showed Grade II reactions. The results of weekly assessment of skin reactions in Group B indicated that the incidence of Grade I skin reactions was seen in 73.3% patients by the end of third week and increased to 83.3% patients by the end of fourth week. By the end of the seventh week, 73.3% patients persisted with Grade I reactions. Similarly, Grade II reactions were seen in 10% patients at the end of the fourth week and increased to 16.6% patients by the end of the fifth and sixth week and decreased to 13.3% patients by the end of seventh week. At the end of one month post treatment, 16.6% patients in Group A remained with Grade I reactions while in Group B, 33.3% patients had Grade I reactions while 6.6% patients persisted with Grade II reactions. These finding are consistent with Mojahed MM et al., and Vivek RS et al., [23,24]. Albeit, Staar S et al., reported a higher incidence in their study [25].
Dysphagia: In this study, the incidence of acute dysphagia in Group A was maximum by the end of the fifth week (60% patients with Grade I and 40% patients with Grade II). Post treatment evaluation at one month revealed Grade I dysphagia in 26.6% patients while no patient had any remaining Grade II dysphagia. Results in Group B revealed maximum Grade I dysphagia by the end of fifth week (60% patients) while maximum incidence of Grade II dysphagia was seen in the fourth week (40% patients). One month post treatment assessment revealed Grade I dysphagia in 13.3% patients while no patient had any remaining Grade II dysphagia. These finding are consistent with the findings of Mojahed MM et al., and Vivek RS et al., [23,24]. But our findings are in contrast with Medina JA study [19] which reported occurrence of Grade III mucosal toxicity in 85% and acute dysphagia in 50% of the patients [19]. However, Staar S et al., have reported a higher incidence of dysphagia [25] as compared to the standard protocols [26,27] that resonates with our findings.
Xerostomia: Hyposalivation is another independent factor that causes difficulty in chewing and aggravates the inflamed tissue leading to an increased risk for local infection [28]. In our study the incidence of xerostomia was estimated to be Grade I (90%), Grade II (10%) in Group A and Grade I (70%), Grade II (23.3%) in Group B at the end of fifth week. However, the incidence of this complication was same after one month of completion of the treatment. The severity was increased but the morbidity associated with xerostomia was very less due to proper supportive care to all the patients. Meshram SD et al., have reported that in their study [7], 57% patients had Grade II xerostomia and one had Grade III xerostomia. In our study, no patients had Grade III xerostomia in either arm.
CBT as a fractionation regimen gives beneficial results by decreasing the number of clonogen cells to a considerable extent with acceptable toxicities. Various studies have proven this effect. CBT allows for an aggressive fractionation schedule and limits the volume of normal mucosa exposed to twice daily radiation therapy (only during last ten fractions in our study). This is of particular significance in HNC [5]. Although, assessment of treatment outcome was not the aim of this study, on one and three month post treatment follow up, we did not find any difference in loco regional control between the two arms. The same can be addressed on a long term basis before arriving at a definite conclusion.
Limitation
This study has its limitations with respect to the small cohort size (n=30 in each arm). In addition, the patients were delivered treatment on cobalt-60 machine that is prone to provide higher toxicities as compared to a linear accelerator (LA), owing to technical limitations. Using LA, the normal tissues can be better prevented from the radiation toxicities. Most importantly, the comparison in disease control between the two arms remains unanswered as of now that would be an important decisive factor. We recommend similar studies in a large sample, prospectively randomized in manner preferably using a LA, and addressing different subsites separately to achieve a better understanding. We hope to formulate guidelines for treating HNC in high volume centres.
Conclusion
It appears worthwhile to conclude that in this prospective study with a 1:1 accrual in each arm, the toxicities in the CBT arm were slightly more (two patients with Grade III mucosal toxicity, more patients with Grade II skin reactions and xerostomia) but did not differ in a statistically significant manner from those in the CF arm. The findings of this study suggest an acceptable toxicity with CBT and along with the advantage of shorter overall treatment time. It may prove to be a beneficial schedule for the management of HNC in high volume centres.