Significant changes in carbohydrate metabolism occur in pregnancy with development of a predisposition for diabetes especially in the third trimester due to the pregnancy hormones especially human Placental Lactogen (hPL) causing insulin resistance [1]. The physiological insulin resistance is important for increased glucose transport across the placenta for foetal energy requirements [2]. However, in many women this hyperglycaemia is exaggerated beyond the upper limits of euglycaemia in pregnancy, labeled as Gestational Diabetes Mellitus (GDM). The prevalence of GDM has been reported to be from 7.8% to 35% depending upon population studied [3,4] and diagnostic criteria used. It can be responsible for many of the adverse fetomaternal outcomes during pregnancy and is also associated with long term health related consequences for both mother and child. Besides genetic predisposition, there are some non-modifiable risk factors i.e., age, ethnicity etc., for the development of diabetes. Studies have linked vitamin D deficiency, a modifiable factor with abnormal glucose metabolism and diabetes [5-10].
The most important forms of vitamin D are vitamin D3 (cholecalciferol) and vitamin D2 (ergocalciferol). Vitamin D3 is produced in skin by exposure to sunlight and vitamin D2 is taken up with fortified food or supplements. Both are hydroxylated in liver to its metabolites (25-hydroxy vitamin D3) and then in kidneys to the active calcitriol (1,25-dihydroxy vitamin D3).
25-hydroxy vitamin D3 has a half life of three weeks and is biologically inactive with levels approximately 1000 times greater than the circulating 1,25-dihydroxy vitamin D3. Therefore, 25-hydroxy vitamin D3 is measured clinically and is the most reliable marker of vitamin D status. As hypovitaminosis D can be easily treated by supplementing vitamin D and may improve the fetomaternal outcome associated with GDM. Therefore, it is pertinent to identify vitamin D as a risk factor for GDM. We therefore, plan to study vitamin D status in pregnant women and to find out its association with GDM.
Materials and Methods
The study was conducted at Hindu Rao Hospital and associated NDMC Medical College, Delhi, India. Approval from Institutional Ethical Committee was obtained. Pregnant women were enrolled at 24-28 weeks gestation from antenatal clinic, after exclusion criteria. Informed written consent was taken from all the participants.
Seventy women were enrolled, out of which only 54 women could be followed up till delivery and were included for the analysis.
Exclusion criteria: Pre-gestational diabetes, hypertension, renal disease, hepatic disease, bad obstetric history, two-hours 75 gm post glucose level ≥200 mg/dL or overt diabetes.
Demographic details of all the women were recorded. Serum vitamin D was estimated in venous blood sample by chemiluminescence immunoassay method at 24-28 weeks gestation. Two hour 75 gm post glucose, plasma glucose was estimated in venous blood of pregnant women. A level of 140 mg/dL and more was used for diagnosing GDM (WHO and DIPSI criteria) [11,12]. Those diagnosed with GDM were taken as cases (Group A) and the rest were taken as controls (Group B). In the control group, for those women with high risk factors i.e., obesity, age, family history etc., the test was repeated after four weeks and if turned out to be positive, were excluded from the control group. Women were followed upto delivery and fetomaternal outcome was recorded in a predesigned proforma.
Statistical Analysis
Data was analysed using SPSS software (Version 16.0). Data was expressed as means and percentages. Nonparametric tests (Mann-Whitney, Chi-square) were used for comparison between the groups. A value less than 0.05 was taken as significant.
Results
There were 21 cases in Group A (GDM group) and 33 cases in Group B (control group). Mean age, parity, BMI and basic laboratory parameters were comparable in both the groups. Mean serum vitamin D was lower in GDM group (3.69±1.67 ng/mL) compared to control group (8.80±8.83ng/mL) [Table/Fig-1].
Demographic distribution and baseline investigations.
| Parameter | Group A(n=21) | Group B(n=33) | p-value |
|---|
| Mean age (years) | 23.42±2.69 | 24.181±3.459 | 0.586 |
| Parity: Primipara Multipara | 1011 | 1221 | 0.763 |
| Mean BMI (kg/m2) | 27.185±5.25 | 24.603±4.479 | 0.055 |
| Mean Hb (gm%) | 10.31±0.699 | 10.53±0.86 | 0.4771 |
| Mean TLC (/mm3) | 11,085.71±1,650.8 | 9445.45±2164.0 | 0.005 |
| Mean Platelets (lacs/mm3) | 2.43±0.507 | 2.37±0.536 | 0.539 |
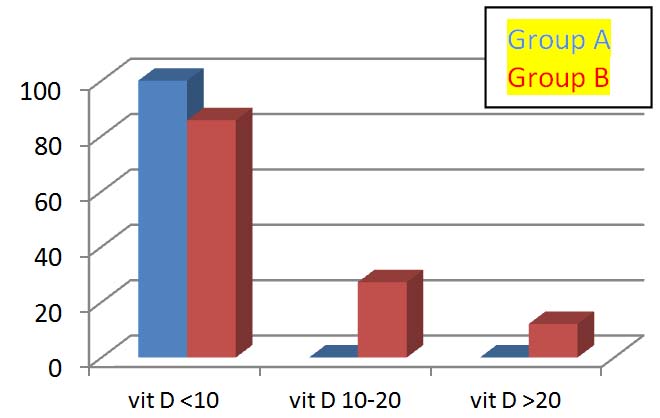
| Mean Vit D (ng/mL)• <10 (deficient)• 10.1- 20 (insufficient)• >20 (optimal) | 3.69±1.6721 (100%)0 (0%)0 (0%) | 8.80±8.8324 (85.7%)5 (15.1%)4 (12.1%) | 0.0040.655-- |
| Mean Vit D (<5 ng/mL)(severe deficiency) | 2.970±0.54618/21 (85.7%) | 3.025±0.08417/33 (51.5%) | 0.4820.018 |
| Mean Plasma glucose (mg/dL) | 143.09±1.757 | 123.575±9.457 | <0.001 |
A significant difference was seen in mean serum vitamin D levels in the two groups (p-value=0.004) [Table/Fig-1]. In Group A, all women had vitamin D less than 10 ng/mL. Out of these, 18 were severely deficient (vitamin D <5 ng/mL) [Table/Fig-1,2].
Women in both groups according to vitamin D levels (ng/mL).
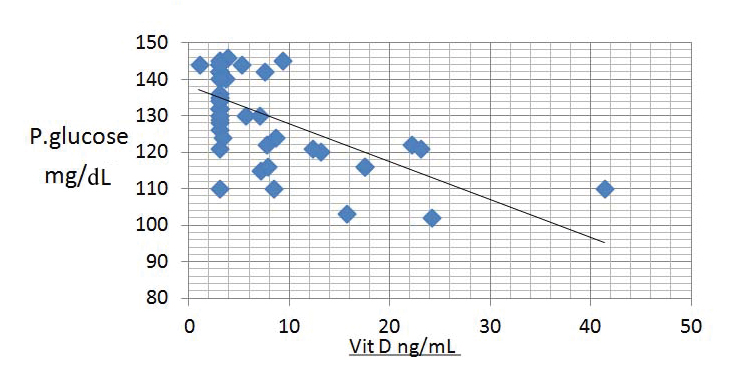
A negative correlation between plasma glucose and serum vitamin D (r-value=0.571) was observed [Table/Fig-3].
Scatter diagram showing association between plasma glucose and serum vitamin D.
Fetomaternal outcome was also evaluated in both the groups. None of the women was diagnosed with overt diabetes (two hours post glucose, plasma glucose levels 200 mg/dL or more) or hydramnios, in GDM group. A significant difference was seen in vitamin D levels in terms of vaginal mode of delivery, birthweight, apgar scores, development of neonatal jaundice and tetany in both the groups [Table/Fig-4]. None of the babies had congenital abnormalities or macrosomia in the two groups.
Fetomaternal outcome and vitamin D in both the groups.
| Outcome | Group A | Group B | p-value(Mann-Whitney) |
|---|
| Mean Vit D | (n) | Mean Vit D | (n) |
|---|
| Mode of deliveryVaginalLSCS | 3.64±1.903.26±0.46 | 183 | 9.87±9.255.438±6.757 | 258 | 0.0010.795 |
| Birth weight<2500 gm≥2500 gm | 3.00±0.003.62±1.81 | 120 | 3.00±0.009.176±8.98 | 231 | --0.005 |
| Apgar≤7 at 1 min>7 at 1 min | 4.57±3.13.36±1.32 | 417 | 3.00±0.008.98±8.90 | 132 | 0.6170.004 |
| Neonatal jaundicePresentAbsent | 3.908±2.2143.08±0.236 | 138 | 12.709±11.7046.484±6.44 | 2211 | 0.1850.003 |
| Neonatal tetanyPresentAbsent | 3.00±0.003.62±1.8 | 120 | 3.00±0.0010.09±9.29 | 627 | --<0.001 |
Discussion
Serum 25 (OH) D levels was estimated in 54 consecutive pregnant women fulfilling the exclusion criteria at 24-28 weeks. We analysed its association with GDM.
In our study, 93% (50/54) of pregnant women were found to be having deficient/insufficient serum vitamin D (<20 ng/mL) [Table/Fig-1]. This is similar with the prevalence rates reported by Sachan A et al., who have reported a prevalence of 84.3% urban and 83.6% in rural area [15]. Marwaha RK et al., in Delhi [16] and Harinarayan CV et al., in Tirupati [17] reported 96.3% and 92.5% prevalence respectively in their studies. Sahu M et al., showed a prevalence of 74.1% from north-eastern part of India [18]. As hypovitaminosis D has adverse fetomaternal effects in pregnancy i.e., development of pre-eclampsia and GDM in mother, increased risk of seizure, jaundice in the neonate and rickets in childhood; it is imperative to supplement vitamin D in antenatal women with hypovitaminosis D.
Distribution of women in the GDM group and controls studied in terms of age, parity and BMI was found to be comparable (p-value 0.586, 0.763 and 0.055, respectively) [Table/Fig-1]. These factors are known to be associated with the development of type 2 diabetes mellitus.
Baseline lab parameters i.e., haemoglobin and platelet count were found to be comparable in both the GDM and control groups.
In our study, out of 54 women analysed, 38.8% (n=21) women developed GDM whereas 61.2% (n=33) women were normoglycaemic. None of the women was diagnosed to be overt diabetic as per DIPSI/WHO criteria. Muthukrishnan J et al., included 70 consecutive women in his study and found 59 women having GDM detected by DIPSI/WHO criteria [6,11,12].
Mean vitamin D level in GDM group was much lower (mean 3.69±1.67 ng/mL) compared to non-GDM group (mean 8.80±8.83 ng/mL). The difference of vitamin D in the two groups was found to be significant in our study (p-value was <0.004). Similar observations of lower vitamin D levels in GDM women have been made by Soheilykhah S et al., Zuhur SS et al., Zhang MX et al., Jain M et al., [7-10].
When the subgroups of vitamin D levels were analysed, it was observed that all the women (100%) in GDM group had vitamin D levels less than 10 ng/mL compared to 85.7% in control group. In Group B 15.1% women had serum vitamin D between 10-20 ng/mL and 12.1% had optimal levels of vitamin D (serum vitamin D >20 ng/mL) [Table/Fig-1,2]. El Sahgeer GM et al., has also made similar observations that all the 40 GDM and 20 pregestational diabetes women in his study had suboptimal vitamin D levels [5].
The frequency of GDM in our study was not significantly different in women with vitamin D deficiency, insufficiency and with optimal levels (p-value=0.655); these results are in corroboration with study by Zuhur SS et al., [8].
In Group A, 85.7% women belonged to severe vitamin D deficiency group (vitamin D levels <5 ng/mL) compared to only 51.5% women in Group B [Table/Fig-1]. The difference in number of women in GDM and control group with severe vitamin D deficiency was found to be significant (p-value=0.018). Thus, it can be said that severe vitamin D deficient women had a higher risk of developing GDM. The risk ratio of developing GDM in severe vitamin D deficiency was 5.647 which is an important observation of our study. A 3.9 fold risk of GDM has been reported in severe vitamin D deficient women by Zuhur SS et al., [8].
An odds ratio of 2.2 and 1.49 has been reported in GDM women by Burris HH et al., and Aghajafari F et al., respectively [17,18]. Soheilykhah S et al., has reported that women with GDM had a 2.66 fold increased risk of vitamin D deficiency (<20 ng/mL) [7]. An 11 times increased incidence of GDM in women with hypovitaminosis D has been reported by Jain M et al., [10]. However, Makgoba M et al., and Pleskacova A et al., did not report any significant association of vitamin D with development of GDM [19,20].
A negative correlation between plasma glucose levels and vitamin D levels (r-value=0.571; Spearman correlation) was seen in our study [Table/Fig-3]. Similar observation was made by Burris HH et al., [17]. Extrapolating this observation it can be said that, improving vitamin D status of a woman might be helpful in decreasing the risk of GDM. Further studies are required to know the role of supplementation of vitamin D in this regard.
The biochemical mechanism in development of GDM in women with hypovitaminosis D can be explained by that vitamin D enhances expression of insulin gene resulting in high insulin synthesis [21]. It also influences insulin secretion by regulating intracellular calcium levels [22] in insulin sensitive tissues such as muscle and adipose tissues. Vitamin D improves insulin resistance by upregulating the insulin receptors [23]. Hypovitaminosis D by promototing inflammatory response induces insulin resistance, therefore, vitamin D by its modulating effect on the immune system [24], enhaces insulin response.
We have also analysed the fetomaternal outcome in terms of vitamin D status in both the groups. GDM group was found to have adverse fetomaternal outcome. It was found that significant difference was present in number of women having vaginal delivery (p-value=0.001), birth weight >2500 gm (p-value=0.005), Apgar >7 (p-value=0.004), absence of jaundice (p-value=0.003), and absence of tetany (p-value 0.000) [Table/Fig-4]. Improving vitamin D status may help reduce these adverse fetomaternal outcomes associated with GDM, needs further evaluation.
Conclusion
Vitamin D was significantly low in women with GDM. Women who had hypovitaminosis D were associated with higher plasma glucose levels. Women with severe deficiency have a 5.6 times risk of developing GDM.