Intravesical BCG is an effective immuno therapy for the treatment of HR NMIBC, which together with Transurethral Endoscopic Resection of Bladder Tumour (TURBT) is considered to be the standard treatment regimen [2]. Approximately, 16% to 40% of patients receiving BCG therapy report recurrence of the disease and around 0.4% to 40% of patients report disease progression [4].
Several fibrin clot inhibitors and Non Steroidal Anti-Inflammatory Drugs (NSAIDs) including aspirin have been examined to improve the efficacy of intravesical BCG in patients with HR NMIBC and few studies have showed positive effect in the prevention of recurrence [5,6]. However, a recent systematic review had showed that there is no strong evidence to support the claim of a protective role of Acetylsalicylic Acid (ASA) [7]. Number of patients with bladder cancer may have concomitant cardiovascular disorder and could be receiving aspirin [8,9].
To our knowledge, there is no clear consensus on the role of NSAIDs along with BCG therapy in patients with HR NMIBC. Hence, this study was undertaken to evaluate the role of aspirin in patients receiving intravesical BCG therapy for HR NMIBC.
Materials and Methods
This was a prospective, single centre, observational study conducted at Department of Urology, RG Kar Medical College, Kolkata, West Bengal, India. This study was conducted over a 24 month period from February 2015 to January 2017, during which consecutive patients of either sex, aged >18 years with a diagnosis of HR NMIBC after TURBT were enrolled. Patients were screened for eligibility based on clinical, radiological and risk factor profile assessment, history of treatment with aspirin and medical records. Patients with absolute and relative contraindications of BCG therapy (immunosuppression, gross haematuria, history of tubercular sepsis, trauma during catheterization, liver dysfunction, etc.,); BCG relapsing, BCG refractory and BCG resistant bladder tumour; patients on NSAIDs or fibrin clot inhibitors other than aspirin before TURBT were excluded.
The study protocol was approved by the Institutional Ethical Committee of the institute. The study was performed in accordance with the approved protocol (OG/WBUHS/2015-16/0311) and ethical standards that have their origin in Declaration of Helsinki 1964, as revised in 2013. Written informed consent was obtained from each patient for participation in the study.
If eligible, patients were divided into two groups based on current aspirin treatment. Patients taking aspirin for a minimum of three months were included in Group 1. Patients not receiving aspirin were included in Group 2. Both groups had received similar induction (120 mg weekly, six cycles) and maintenance course (120 mg weekly for three weeks at three months, six months, 12 months, 18 months) of intravesical BCG (Oncovac, Danish 1331 strain) as per SWOG schedule [10]. During follow up period, cystoscopy was done every three months, as per European Association of Urology (EAU) guideline [2]. Study outcomes were recurrence and progression of bladder tumour. Recurrence was defined by visual and/or biopsy proved bladder tumour in the follow up cystoscopy (every three months) and progression was defined by the upgrading of the tumour stage.
Statistical Analysis
No formal sample size calculation was employed for this study. All statistical analysis were conducted using SPSS version 20.0 (IBM, Armonk, NY). All reported p-values were two-sided and considered statistically significant when p<0.05. Associations between predictive factors and recurrence or progression (stage progression) were evaluated using Chi-square and Fisher’s-exact test to test for statistical significance. Wherever the expected counts in at least one cell were <5, Fisher’s-exact test was used to calculate significance. In all others, Chi-square test was used. Binary logistic regression was performed to ascertain the effect of variables (age, sex, smoking and aspirin intake, type of the tumour, focality, grade and muscle in specimen and tumour stage) to predict the recurrence or progression (except sex for progression). Recurrence and progression free survival rates were evaluated using Kaplan-Meier methods.
Results
During the study period, a total of 152 patients were diagnosed with HR NMIBC, of which 103 patients who had fulfilled the inclusion and exclusion criteria were included in the study. Reason for exclusion of the 49 patients were: upgradation of stage to muscle invasive during initial restage TURBT (n=22), follow up for less than six months (n=8), did not receive full course of induction BCG therapy (n=6), chronic user of other NSAIDs/fibrin clot inhibitors (n=5) and known case of BCG failure and contraindicated BCG (n=4, each).
Included (n=103) patients were studied for a mean follow up period of 10.8 months. The mean (SD) age was 58.0 (9.5) years and ranged from 29 to 85 years. Overall, 90 (87.4%) patients were men and 74 (71.8%) patients were smokers; and the mean (SD) number of pack-years (defined as 20 cigarettes per day for one year) was 15.5 (13.4) and the range was 0-50 [Table/Fig-1].
Baseline demographics and clinical characteristics.
| Parameters | Total n=103 (%) |
|---|
| Mean age (SD) | 58.0 (9.5) |
| GenderMenWomen | 90 (87.4)13 (12.6) |
| Smoking/ tobacco use | 74 (71.8) |
| Aspirin intake | 15 (14.6) |
| SymptomsHaematuriaIrritative LUTSBoth | 94 (91.3)32 (31.1)25 (24.3) |
| Tumour typePapillarySessile | 82 (79.6)21 (20.4) |
| Tumour numberUnifocalMultifocal | 70 (68.0)33 (34.0) |
| StagepTapT1pTcis | 15 (14.6)73 (70.8)15 (14.6) |
| GradeHighLow | 77 (74.8)26 (25.2) |
| Muscle in the specimen | 70 (68.0) |
| Medical historyMyocardial infarction/unstable anginaStroke | 10 (9.7)5 (4.9) |
Data presented as n (%), unless otherwise specified.
LUTS-Lower Urinary Tract Symptoms; SD-Standard Deviation.
Haematuria was reported in 91.3% patients, irritative Lower Urinary Tract Symptoms (LUTS) were reported in 31.1% patients; however, both haematuria and LUTS were reported in 24.3% patients. A total of 70.8% patients had stage pT1 bladder cancer, 74.8% of the tumours were high-grade and most of them were papillary type (79.6%) and unifocal (68%). The muscle was present in the 68% of primary TURBT specimen [Table/Fig-1].
Overall, 15 (14.6%) patients were taking aspirin (75 mg) for more than three months, labelled as Group 1 and rest (n=88) of them as Group 2. Out of total 15 patients, who were taking aspirin, 10 patients had history of myocardial infarction/unstable angina and five patients had history of stroke. Mean (range) duration of aspirin intake was 21.6 (6 to 60) months. There was no statistically significant association between aspirin intake and the tumour staging (p=0.595) or grading (p=0.558). Similarly, there was no statistically significant recurrence (26.7% versus 30.7%, p=0.508) and progression (25% versus 44.4%, p=0.621) between Group 1 and Group 2, respectively [Table/Fig-2].
Association between aspirin and tumour stage, grade, recurrence and progression rate.
| Parameters | Group 1 (n=15) | Group 2 (n=88) | p-value |
|---|
| StageTaT1 Tcis | 3 (20.0)9 (60.0)3 (20.0) | 12 (13.6)64 (72.7)12 (13.6) | 0.595 |
| GradeG1G3 | 4 (26.7)11 (73.3) | 22 (25.0)66 (75.0) | 0.558 |
| Recurrence rate | 4 (26.7) | 27 (30.7) | 0.508 |
| Progression rate | 1 (25.0) | 12 (44.4) | 0.621 |
Data presented as n (%).
Group 1- Patients with aspirin for a minimum of three months; Group 2- Non-aspirin.
p-value was calculated using Chi-square test (Fisher’s-exact test for counts <5).
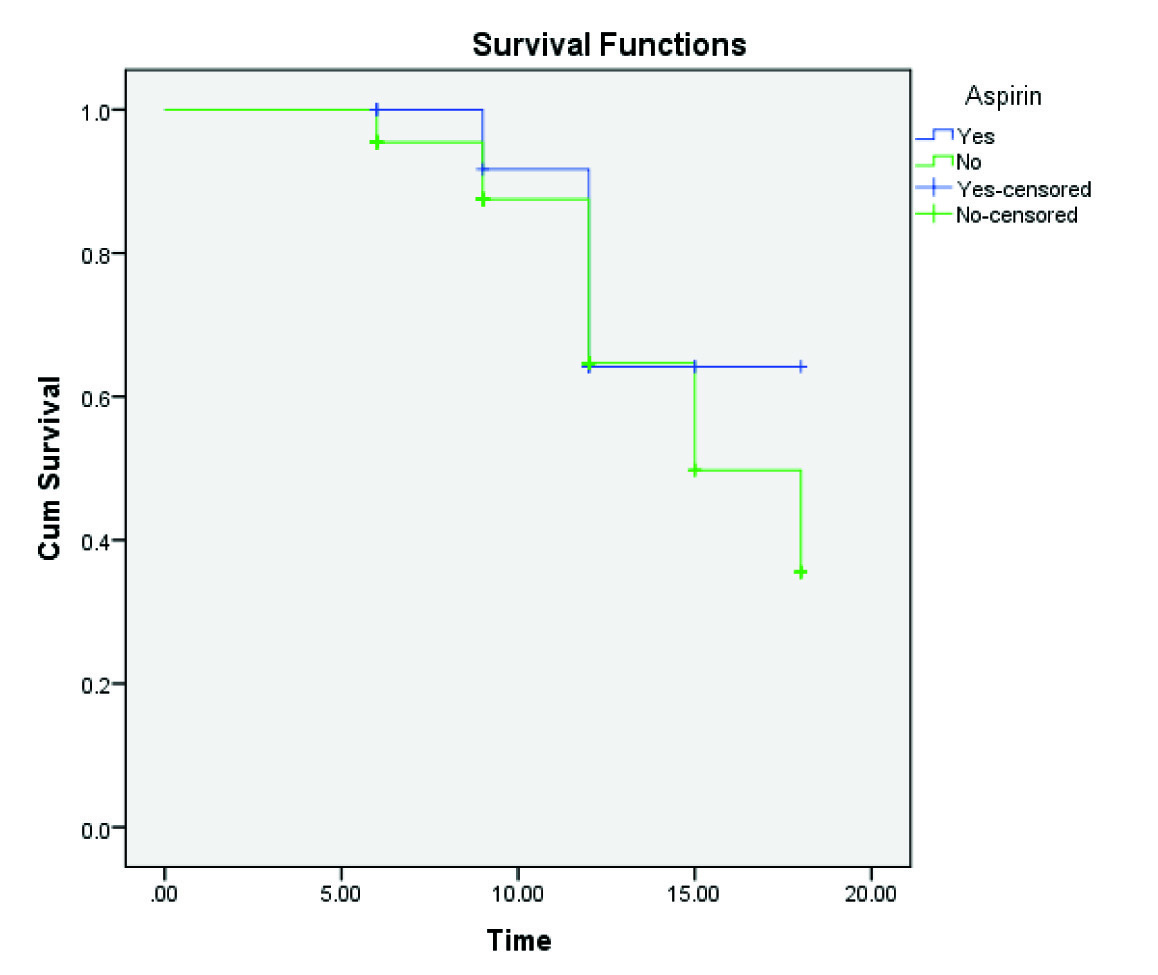
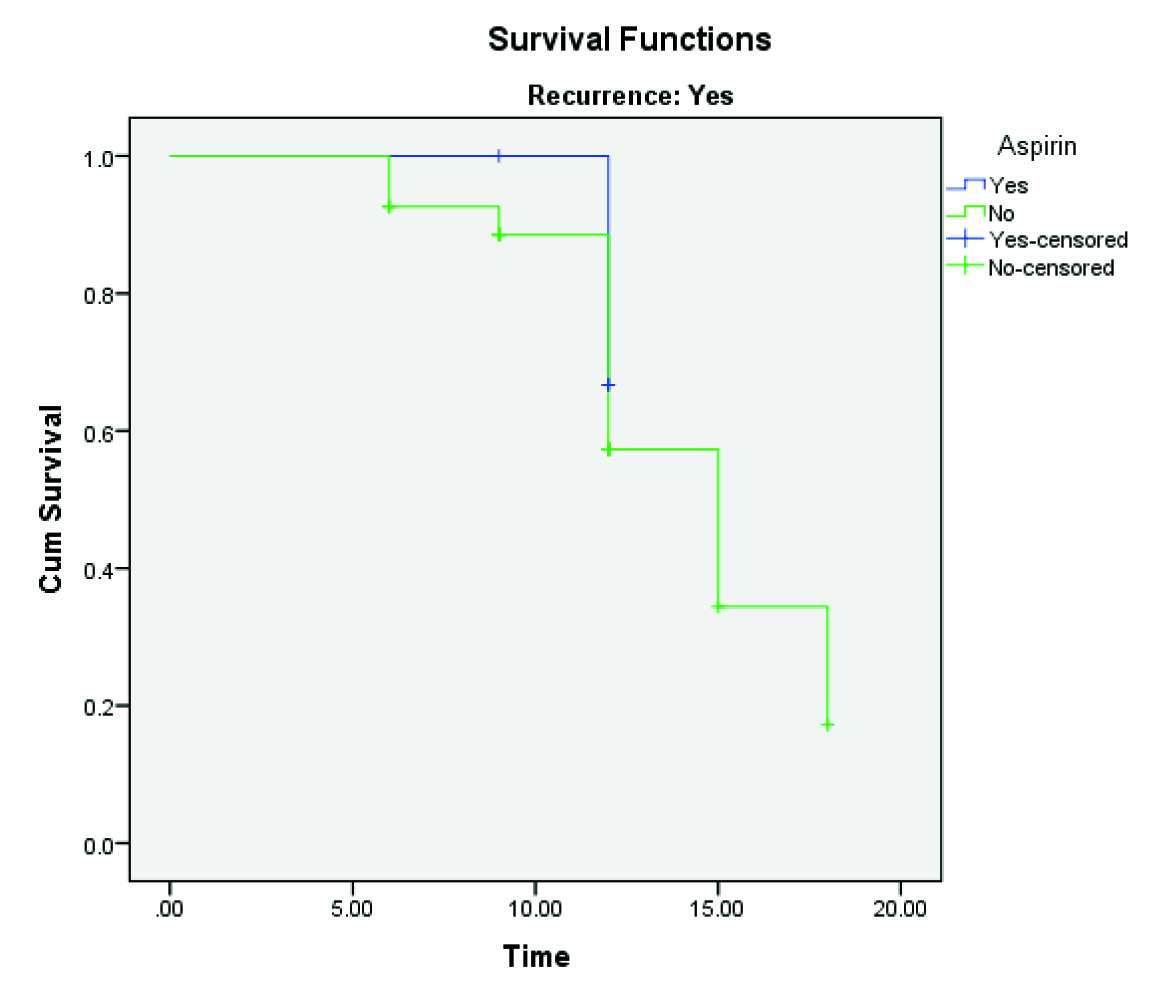
A twelve month Recurrence Free Survival Rate (RFSR) in aspirin group was 64.2% and the non-aspirin group was 64.7% (p=0.566) but 15-month RFSR in aspirin group remained 64.2%, though non-aspirin group decreased to 49.7% and further decrease to 35.5% at 18 months [Table/Fig-3]. Twelve-month Progression-Free Survival Rate (PFSR) in aspirin group was 66.7% and the non-aspirin group was 57.3% (p=0.640), which decreased to 34.4% at 15 months and further decrease to 17.2% in the non-aspirin group at 18 months [Table/Fig-4].
Kaplan-Meier recurrence free survival analysis.
Kaplan-Meier progression free survival analysis.
Binary logistic regression showed none of the variables except focality had a significant role in the prediction of recurrence of bladder cancer (0.001) [Table/Fig-5]. The similar analysis showed that none of the variables were significant in predicting disease progression [Table/Fig-5].
Predictors of recurrence and progression.
| Variables | Recurrence | Progression |
|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value |
|---|
| Age | 0.978 (0.930, 1.029) | 0.400 | 0.925 (0.822, 1.041) | 0.196 |
| Sex | 0.171 (0.019, 1.525) | 0.114 | - | - |
| Smoking | 1.014 (0.977, 1.053) | 0.456 | 1.012 (0.940, 1.090) | 0.298 |
| Aspirin | 1.002 (0.241, 4.164) | 0.997 | 11.65 (0.114, 1188.3) | 0.114 |
| Tumour type | 1.464 (0.457, 4.694) | 0.521 | 7.009 (0.625, 78.618) | 0.086 |
| Focality | 5.064 (1.868, 13.73) | 0.001* | 6.647 (0.767, 57.614) | 0.360 |
| Grade | 1.025 (0.316, 3.319) | 0.968 | 0.281 (0.018, 4.263) | 0.159 |
| Muscle in specimen | 0.674 (0.237, 1.916) | 0.459 | 6.379 (0.483, 84.294) | 0.680 |
| T stage | 0.747 (0.196, 2.849) | 0.669 | 0.588 (0.047, 7.328) | 0.608 |
CI- Confidence Interval; OR-Odds Ratio. *Statistically significant.
Binary logistic regression was performed to ascertain the effect of variables.
Discussion
This prospective observational study conducted in Indian patients with HR NMIBC demonstrated that aspirin may not have a role in predicting recurrence and progression. Despite treatment with BCG after TURBT, 40% of HR bladder cancers recur or progress; hence, it was deemed necessary to examine the factors which could improve the efficacy of BCG. The BCG through activation of natural killer cells and cytotoxic T cells after adhesion to exposed fibronectin initiates an antitumour response.
The antitumour mechanism of aspirin and other fibrin clot inhibitors is possibly through interrupting the clotting mechanism, which prevents tumour cell adherence and implantation [11]. Aberrant expression of cylcooxygenase (COX) enzyme was identified in bladder tumours and its inhibition with COX inhibitors has been proved in bladder cancer models [12,13]. The inhibition of COX enzyme increases interleukin-12 production by dendritic cells after BCG therapy. Since, interleukin-12 promotes T helper 1 cell differentiation and in turn cytotoxic T-cell activation; these data suggest another mechanism by which COX inhibition may enhance the efficacy of BCG therapy. On the contrary, few studies also showed that, fibrin clot inhibitors decrease BCG adherence and the local attraction of monocytes and other immune cells and consequently decrease in the BCG efficacy [14,15].
In a study by Gee JR et al., 43 patients with HR bladder cancer were retrospectively evaluated, of whom 20 patients were taking aspirin. The results showed significantly lower five-year RFSR among patients taking aspirin than patients without aspirin (64% versus 27%, p=0.03) [16]. In another study by Boorjian SA et al., 221 patients with bladder cancer taking atleast one fibrin clot inhibitor were retrospectively evaluated, of whom 170 patients were taking aspirin [17]. The results demonstrated that, patients receiving aspirin had a significantly improved five-year probability of freedom from surgery than those who did not receive aspirin (66% versus 56%, p=0.029).
In contrary, in an analysis of 183 patients treated with BCG, Witjes JA et al., found no significant difference in tumour recurrence among aspirin (n=42) versus non-aspirin group (n=141) (31% vs. 40%, p=0.28) [18]. Singla M et al., by univariate COX regression analysis of 99 patients taking NSAIDs and/or statin, concluded that the use of any anti-inflammatory drugs including aspirin do not predict recurrence, stage progression, progression to cystectomy, overall survival and cancer specific survival [19]. In present study, the recurrence rate among aspirin and a non-aspirin group was not significantly different (26.7% versus 30.7%, p=0.508).
In a study by Boorjian SA et al., who evaluated tumour progression among patients with bladder cancer receiving BCG, found that the disease progression was decreased in patients receiving aspirin (HR 0.71 [95% CI: 0.52, 0.96]; p = 0.024) [17]. In another study, Gee JR et al., found that progression rate was similar between aspirin and non-aspirin groups [16]. In the present study, no significant difference was seen in progression rate among both groups (25.0% versus 44.4%, p=0.621). There was no significant difference between Group 1 and Group 2 for 12 months RFSR and PFSR. In the present study, none of the variables demonstrated any association between variables, recurrence and progression of disease, except for focality to predict recurrence of bladder cancer. In a recent study, Singla N et al., analysed data retrospectively with variables like age, gender, tobacco use, aspirin and statin intake, BMI, focality, stage, the size of the tumour and co-morbidity score but they did not find any significant association between any of these predictor variables for recurrence and progression [19].
To the best of our knowledge, this was the first prospective study conducted to evaluate the role of aspirin in the efficacy of BCG, each patient received induction and maintenance BCG therapy, adherence to aspirin intake was assured during the perioperative period to minimise the bias.
Limitation
The authors also acknowledge several limitations of this study, including shorter duration which limits the assessment of long-term survival and less number of patients in the aspirin group; hence care must be taken when generalising these results. Another limitation was that we did not assess the effect of aspirin among low grade NMIBC or did not have data on the duration of aspirin use prior to enrollment in this study which might affect the recurrence or progression of bladder tumour. Further research is warranted to confirm these gaps.
Conclusion
Results from this study showed that aspirin intake does not affect the short-term oncologic outcome of HR NMIBC in patients receiving BCG therapy. Focality was the significant predictive variable for recurrence of the tumour. Further research with higher sample size and longer duration are needed to confirm these results.
Data presented as n (%), unless otherwise specified.
LUTS-Lower Urinary Tract Symptoms; SD-Standard Deviation.
Data presented as n (%).
Group 1- Patients with aspirin for a minimum of three months; Group 2- Non-aspirin.
p-value was calculated using Chi-square test (Fisher’s-exact test for counts <5).
CI- Confidence Interval; OR-Odds Ratio. *Statistically significant.
Binary logistic regression was performed to ascertain the effect of variables.