Tubercular empyema accounts for majority of empyema cases in the developing world [1,2], in contrast to the scenerio in the developed countries, where non-mycobacterial pulmonary infections and surgical procedures are the commonest cause of empyema thoracis [3]. Clinical course and outcomes of tubercular empyema are complicated because of chronicity of illness, frequent association of pulmonary cavitary lesion, high bacillary load, presence of bronchopleural Fistulae (BPF) and need for thoracic surgical intervention [1,2]. A confident early diagnosis of tubercular empyema remains challenging for the clinicians as microbiological confirmation of Mycobacterium tuberculosis becomes difficult in many cases as evidenced by relatively low yield of Ziehl-Neelsen staining as well as mycobacterial cultures in pus from pleural space in previous studies [1,4-6]. CBNAAT utilizes a nested Polymerase Chain Reaction (PCR) technique to identify small quantities of genetic elements of Mycobacterium tuberculosis from clinical specimens and it has advantage of identifying rifampicin resistance at the same time. CBNAAT is completely automated, has minimal biosafety hazard and can give result within two hours. World Health organisation has endorsed the use of this rapid molecular diagnostic test for diagnosis of tuberculosis with special emphasis on drug-resistant tuberculosis, TB-HIV co-infection, paediatric tuberculosis, extrapulmonary tuberculosis and smear negative pulmonary tuberculosis [7,8]. There has been paucity of data in role of CBNAAT in diagnosis of tubercular pleural effusion and empyema. In this background, the present study was carried out to determine the occurrence, clinical characteristics, diagnostic role of CBNAAT and outcome of patients with tubercular empyema.
Material and Methods
A Prospective study of all adult cases (above 15 years of age) of tubercular empyema admitted in the Department of Respiratory Medicine of Institute of Postgraduate Medical Education and Research in Kolkata was carried out over a period of 18 months (January 2015-June 2016).
Patient Selection
Case definition: Tubercular empyema was defined as cases of thoracic empyema with one of the following: (1) pleural fluid positive for Acid Fast Bacilli (AFB) smear or positive for Mycobacterium tuberculosis on CBNAAT; and/or (2) sputum positive for AFB or positive for Mycobacterium tuberculosis on CBNAAT and having radiological lesions consistent with active parenchymal tuberculosis on chest X-Ray/CT scan of the thorax (nodular consolidation with or without cavity in apex, tree in bud appearance). Probable tubercular empyema was defined as empyema in patients who had radiological evidence of active pulmonary tuberculosis on chest X-Ray/ CT scan of the thorax or were sputum positive for AFB smear or by CBNAAT [1]. Written informed consent was taken from all patients and the study was cleared by the Institute’s Ethical Committee.
Exclusion criteria: 1) age less than 15 years; 2) non-tubercular empyema cases; 3) empyema secondary to penetrating or blunt chest trauma; 4) empyema secondary to any surgical procedure; 5) patients not giving consent for the study.
Study protocol: Thorough evaluation of clinico-demographical parameters such as age, sex, symptoms with duration, co-morbidities (diabetes mellitus, HIV infection) were evaluated in all patients who fulfilled the case definition and had consented for the study. Chest skiagrams (CXR) were done in all patients on admission. Follow-up CXR were done following insertion of Intercostal Tube Drain (ICTD), before removal of ICTD, on discharge and when felt necessary in- between. Ultrasound and Computed Tomography (CT) of thorax were also done when needed. Pleural fluid was aspirated under aseptic conditions by thoracentesis. If aspirated fluid was frank pus macroscopically, it was sent only for Gram staining, culture (aerobic and anaeobic), smear for AFB by Ziehl-Neelsen stain and CBNAAT (Cepheid,GX-IV Processing Unit: 11.00" w x 12.00" h x 11.70" d, GXIV-4-D) for Mycobacterium tuberculosis [7,9]. When pleural fluid did not appear to be frankly purulent on aspiration, in addition to the microbiological tests mentioned above, pleural fluid were also studied for Total Leucocyte Count (TLC), differential leucocyte count, protein, sugar and Lactate Dehydrogenase (LDH). Although, measurement of Adenosine Deaminase (ADA) is useful in distinguishing tubercular effusion from other causes of exudative effusion, it is elevated in empyema of any aetiology; hence, estimation of ADA was not done in our cases. Pleural fluid mycobacterial cultures were not included in this study protocol because of its cost and reports of low sensitivity of mycobacterial cultures in tubercular empyema by previous studies [1,6,10-12]. Sputum for AFB smear and CBNAAT for Mycobacterium tuberculosis were done in all cases. Relevant investigations like complete blood counts, renal and liver function tests, blood for HIV serology from Integrated Counseling And Testing Centre (ICTC), blood sugar (fasting and post–prandial) were also done in all patients. Subsequently, only those cases who fulfilled case definition of tubercular empyema after obtaining the investigation results were recruited for the study and analysed further.
Diagnosis of bronchopleural fistula (BPF) was considered if chest X-Ray on admission(prior to thoracentesis) revealed horizontal air-fluid level in the upright position and if there was persistence of bubbling/air leak through the tube thoracostomy even after 24 hours of intercostals tube drainage [2].
Regarding drainage of pus, closed Intercostal tube drainage (ICTD) was done with a straight chest tube without trocar (Romson 28G to 32 G) with a water-seal drainage system. Continuous drainage was maintained until fluid was serous, daily collection was less than 50 ml, pleural cavity was obliterated by expansion of the lung and any BPF was sealed. In cases of multiloculated and small empyemas where ICTD or pigtail catheter insertion could not be done, serial ultrasound-guided aspiration of pleural pus was done. All patients of tubercular empyema were put on appropriate category of antitubercular drugs as per current Revised National Tuberculosis Control Program (RNTCP) protocol [13]. Intrapleural fibrinolytics was not used in this study based on the results of Multicenter Intrapleural Sepsis Trial (MIST) [14]. Thoracic surgical procedures like decortication, decortication with closure of bronchopleural fistula using intercostal muscle flap were considered by thoracic surgeon in patients who failed to respond to antitubercular drug therapy and ICTD, as evidenced by persistence of air leak/BPF and radiological non-expansion or partial expansion of lung.
Outcome: Patients were followed up for three months. Outcome was defined as one of the following: Cure-complete expansion of lung with acceptable pleural thickening of less than two cm in chest X-ray PA view; Failure-it was defined as recurrence or persistence of BPF even after medical and surgical management; Death-death occurring due to the disease process during the study period.
Statistical Analysis
Statistical analyses were performed using SPSS version 20.0 (SPSS inc., Chicago, IL) software for MS-Windows. Descriptive frequencies were expressed using mean and standard deviation. Sensitivity and specificity were calculated with 95% Confidence Interval (CI) where relevant.
Results
A total of ninety five empyema cases were admitted during the one and half year study, of which fifty five were cases of non-tubercular empyema and were excluded from this study. Tubercular empyema was diagnosed in 40 cases (40 out of 95, 42.1%), these 40 cases of tubercular empyema were recruited in this study and were analysed further.
Aspiration of frank pus was found in 33 cases of tubercular empyema (82.5%). Majority of tubercular empyema was seen in males (29 out of 40, 72.5%). Mean age was 31.5 years in the tubercular empyema group (range: 16-68 years). Thirty five cases (87.5%) of tubercular empyema had a chronic illness of more than one month. Cough, fever and shortness of breath were the commonest symptoms in the study population. BPF were found in 17 (42.5%) cases [Table/Fig-1]. Diabetes mellitus was found in 15 patients (37.5%) and associated Human Immunodeficiency Virus (HIV) co-infection was seen in 3 patients (7.5%). Routine blood investigations showed mean values of haemoglobin concentration, total leucocyte count, erythrocyte sedimentation rate and serum albumin were 9.6 gram%, 10027/cubic millimeter, 72 at the end of first hour and 2.9 gram/decilitre respectively. In those seven cases, where pleural fluid was not frankly purulent, pleural fluid total leucocyte count showed a mean value of 1942.85/cubic millimeter {±standard deviation (SD)-977.84} with a neutrophil predominance {neutrophil-mean 81.14% (±9.45 SD); lymphocyte-mean 20.36% (±7.73 SD)}. Mean pleural fluid sugar was found to be16 mg/deciliter (SD-7.04) and mean LDH was 4348.17 units/litre (SD-2718.73).
Clinical characteristics of Tubercular empyema.
| Tubercular empyema(n=40) |
|---|
| Age in years mean, [SD] (range) | 31.5 [8.45], (16-68) |
| Number of patients with duration ofIllness more than one month | 35 (87.5%) |
| Cough | 40(100%) |
| Fever | 35 (87.5%) |
| Shortness of breath | 38 (95%) |
| Hemoptysis | 9 (22.5%) |
| Empyema Necessitans | 5(12.5%) |
| Bronchopleural fistula (BPF) | 17 (42.5%) |
Radiology
Twenty seven patients (67.5%) of tubercular empyema had presented with pyopneumothorax on admission. Concommittant parenchymal lesion suggestive of pulmonary tuberculosis in the ipsilateral or contralateral lung was found in 24 (60%) patients [Table/Fig-2]. Consolidation (24 cases, 60%) and cavity (7 cases, 17.5%) were the common parenchymal lesions on CXR with presence of bilateral lesion in 11 cases (27.5%).
Chest X-Ray PA view showing left sided pyopneumothorax with parenchymal lesion on right upper zone.
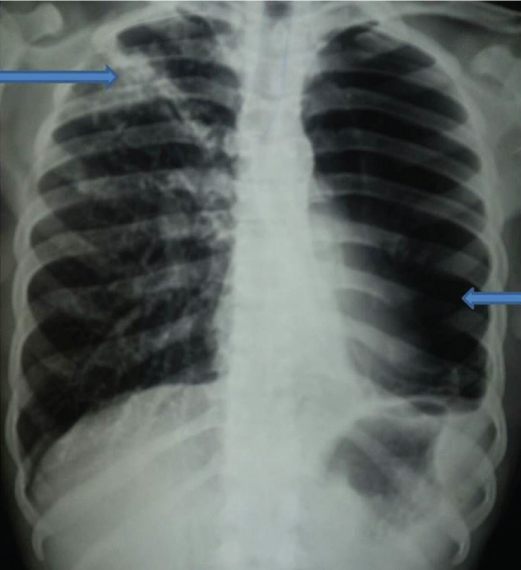
Microbiologic Diagnostic Spectrum
Sputum for AFB smear was positive in 22 cases (55%) and sputum CBNAAT detected Mycobacterium tuberculosis in 23 cases (57.5%) [Table/Fig-3]. Pleural fluid AFB smear was positive in 29 cases (72.5%) and on pleural fluid CBNAAT, Mycobacterium tuberculosis was detected in 37 cases (92.5%) cases [Table/Fig-3]. Both sputum and pleural fluid smear or CBNAAT were positive in 20 cases (50%). Pleural fluid Gram staining was also positive in seven of the proven tubercular empyema cases but culture was negative in all these cases.
Microbiological Diagnosis of Tubercular Empyema (n= 40).
| Pleural fluid AFB smear positive | 29 (72.5%) |
| Pleural fluid CBNAAT positive | 37 (92.5%) |
| Sputum AFB smear positive | 22 (55%) |
| Sputum CBNAAT positive | 23 (57.5%) |
To determine the combined diagnostic efficacy of pleural fluid AFB smear and pleural fluid CBNAAT, it was found that 95% patients (n=38) of tubercular empyema showed positive result on either pleural fluid CBNAAT or AFB smear, both pleural fluid AFB smear and CBNAAT were positive in 70% cases; and in two patients of tubercular empyema, both pleural fluid AFB smear and CBNAAT were found to be negative [Table/Fig-4]. Both the cases were positive on sputum for AFB smear and CBNAAT, and radiological lesions were also consistent with pulmonary tuberculosis in these two patients. Rifampicin resistance was detected in one de novo patient in pleural fluid CBNAAT, sputum for CBNAAT also showed detection of Mycobacterium tuberculosis and presence of Rifampicin resistance in that patient.
Diagnostic yield of Pleural fluid AFB smear and CBNAAT (n=40).
| Pleural fluid AFBsmear positive (n=29) | Pleural fluid AFBsmear negative |
|---|
| Pleural fluid CBNAAT positive (n=37) | 28 (70%) | 9 (22.5%) |
| Pleural fluid CBNAAT negative (n=3) | 1 (2.5%) | 2 (5%) |
Overall, when pleural fluid CBNAAT result was compared with a composite diagnostic standard of tubercular empyema (clinicoradiological+ pleural fluid and/or sputum AFB smear ± histopathology), diagnostic sensitivity and specificity of pleural fluid CBNAAT were found to be 92.5% and 100% respectively [Table/Fig-5]. Sensitivity of pleural fluid CBNAAT was 96.5% in cases where pleural fluid AFB smear was also positive, but in pleural fluid AFB smear negative cases, sensitivity of pleural fluid CBNAAT was 81.8%.
Sensitivity and specificity of Pleural fluid CBNAAT in tubercular empyema.
| Tubercular Empyema (Composite diagnostic index) (n=40) | Non-tubercular empyema (n=55) |
|---|
| Pleural fluid CBNAAT positive | 37 | 0 |
| Pleural fluid CBNAAT negative | 3 | 55 |
*Sensitivity-92.5% (95% CI 79.61- 98.43); Specificity-100% (95% CI 93.51-100.00)
Treatment and Outcome
Twelve patients (30%) had a past history of intake of antitubercular drugs (ATDs), and seven (58.3%) of these twelve patients had a past history of antitubercular treatment default. These patients were started on category II ATD under RNTCP. Rifampicin resistance was detected in both pleural fluid and sputum CBNAAT in a new patient and she was put on category IV ATD as per RNTCP DOTS-PLUS protocol. The remaining 27 cases were newly diagnosed cases of tuberculosis and were put on category I ATD [13].
As regard to drainage of pus, Intercostal tube drainage under local anaesthesia was the preferred drainage modality and was carried out in 35 (87.5%) of the tubercular empyema cases. Ultrasound (USG) guided drainage of pus was the modality of drainage in the remaining five patients where Intercostal tube drainage was not possible due to multiple loculation. Twenty nine (72.5%) patients needed ICTD for prolonged duration (> one month) and mean duration of ICTD was 45.6 days in the study group. Eighteen patients (45%) needed thoracic surgery as there was failure of lung expansion and/or persistence of BPF in spite of ATDs and ICTD [Table/Fig-6]. Six of these eighteen patients needed decortication only, and rest 12 patients had undergone decortication with BPF closure [Table/Fig-7].
Treatment modalities and outcome.
| Tubercular empyema (n=40) |
|---|
| Mean duration of ICTD | 45.6 days |
| No. of patients with ICTD > 1 month* | 29 (72.5%) |
| Surgical drainage* | 18 (45%) |
| Failure of lung re-expansion after surgery | 4 (10%) |
| Death | 1 (2.5 %) |
Peroperative photograph during decortication showing thick pleura.
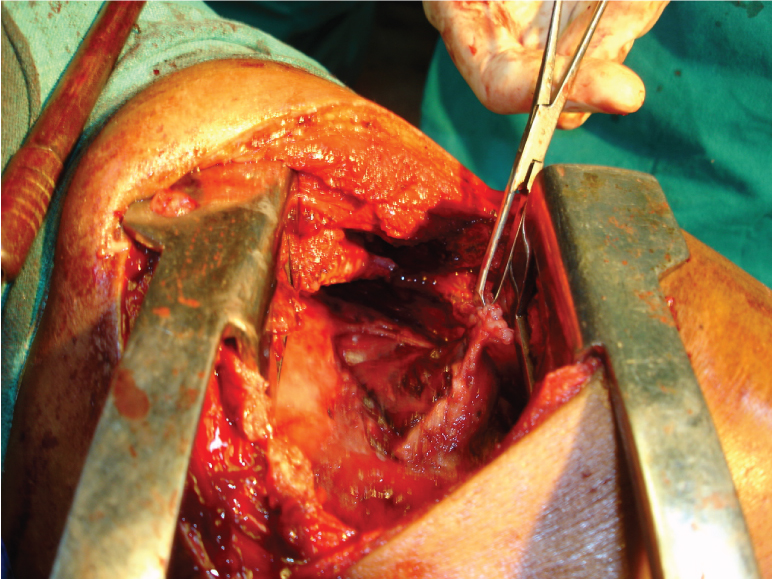
The outcome was unfavourable in five cases (12.5%) with failure of lung expansion after surgery in four cases and death in one case as patient succumbed from extensive pulmonary parenchymal disease.
Discussion
Empyema thoracis is an important cause of infectious disease related morbidity and mortality in developing countries [1,2]. During the study period, a total no. of ninety five empyema cases were encountered, of which 40 patients fulfilled the criteria of tubercular empyema. In contrast to western literature, Indian studies have shown that tubercular empyema constitutes a large no. of cases, ranging from 38.6% to 65%, of empyema cases [4,5,10,15,16]. This study also showed tubercular empyema accounted for 42.1% of total empyema cases.
Mean age of tubercular empyema was 31.5 years in this study with a predilection for younger population, 45% being less than 30 years of age. Similar results have been shown in studies by Kundu et al., [2], Acharya et al., (commonest age group for tubercular empyema 20-40 yrs) and Goyal et al., (commonest age group for tubercular empyema 21-40 yrs) [10,15]. This can be explained by high prevalence of pulmonary tuberculosis in the young active age group, as has been documented in our country [17,18]. This may probably be due to the fact that, young active males work more at outdoors in India and more frequently come in contact with sputum positive pulmonary tuberculosis cases; secondly, this young active males are also the commonest age group for HIV infection and that synergistically increases chance of active tuberculosis in this age group. A male preponderance (72.5%) was noted in tubercular empyema cases in this study. Other Indian studies such as Malhotra et al., (75%), Kundu et al., (71.4%), and Sethy et al., (87%) have also shown a male predominance among tubercular empyema cases [1,2,11]. As previous studies on tubercular empyema, diabetes mellitus was found to be the commonest comorbid illness in this study [1,2,19]. Presence of frequent BPF (42.5%) and empyema necessitans (12.5%) intubercular empyema have also been supported by other studies [1,2,19].
Many previous Indian studies on tubercular empyema have shown a low percentage of bacteriological positivity in sputum and pleural fluid [4,5], but it has to be kept in mind that those studies were mostly done before availability of good quality pleural fluid mycobacterial cultures and rapid molecular tests like CBNAAT. In this study, pleural fluid smear for AFB was positive in 72.5% (29cases) and pleural fluid CBNAAT detected Mycobacterium tuberculosis in 92.5% (37 patients) and combined diagnostic yield of pleural fluid AFB smear and CBNAAT was 95%. Sputum smear and/or CBNAAT was positive in 57.5% (23 cases) and both sputum and pleural fluid smear for AFB were positive in 50% (20 cases). Kundu et al., have also reported a high percentage of pleural fluid AFB smear positivity (93.1%) and sputum AFB smear positivity (65.5%) in their study [2]. Malhotra et al., Prakash B et al., and Goyal et al., also reported 57%, 71.69% and 48.8% pleural fluid AFB smear positivity respectively [1,6,15]. High percentage of both sputum and pleural fluid AFB smear positivity in these Indian studies can be attributed to pathogenesis of tubercular empyema, where usually a tubercular cavity ruptures into the pleural space giving rise to tubercular pyopneumothorax with high bacillary load [16,20,21].
In contrast to, usual low yield of pleural fluid CBNAAT in diagnosis of tubercular pleural effusion, pleural fluid CBNAAT was found to be positive in 92.5% cases in this study with a specificity of 100%. Sensitivity of pleural fluid CBNAAT was good in both pleural fluid AFB smear positive cases (96.5%) and pleural fluid AFB smear negative cases (81.8%). Similar high sensitivity of CBNAAT in pus samples (87.6%) and low sensitivity (44.4%) in tubercular pleural effusion have been reported by Lawn SD and Zumla AI [22]. This can again be explained by the difference in pathogenesis between tubercular empyema and tubercular pleural effusion, while the former has a high bacillary load, the later entity is pauci-bacillary [20,21,23,24]. Another advantage of CBNAAT is its ability to identify rifampicin resistance at the same time. In our study, one patient showed primary Rifampicin resistance in both pleural fluid and sputum CBNAAT. Concomitant bacterial superinfection was evidenced in seven cases (as evidenced by associated positivity on Gram stain but culture was negative in all those cases). Other studies have also reported incidence of bacterial co-infection in cases of tubercular empyema [1,6]. Bacterial co-infections may be explained by presence of BPF or complication of interventions like intercostal tube drainage.
Limitation
In this study, patients required prolonged intercostal tube drainage (mean duration-45.6 days), 45% required thoracic surgery and even after that 12.5% had an unfavourable outcome. Kundu et al., showed in their study that mean duration of ICTD, was 48.7 days, thoracic surgery was necessary in 55.2% and poor outcome was noted in 17.2% cases [2]. These findings have been also supported by other Indian studies [6,9,14,15]. In several previous studies, mortality in tubercular empyema ranged from 8%-50% [1,5,6,15], but in this study it was low (2.5%). Kundu et al., have also reported a low mortality rate (3.45%) in tubercular empyema [2]. Low mortality rate in this study may be attributed to rapid diagnosis by CBNAAT, strict adherence with RNTCP protocol based antitubercular drug therapy and a timely drainage of pus. Prolonged duration of illness, concomitant bronchopleural fistula were found to be related to unfavourable outcome [1,25,26].
Sample size of this study was relatively small, so future studies, preferably multicentric with larger sample size are required to establish the findings of this study more firmly. Further, studies in this field comparing yield of CBNAAT versus mycobacterial culture will also be helpful.
Conclusion
Tubercular empyema is a fairly common entity in our country especially in the young population. It is frequently complicated by bronchopleural fistula, often requires complicated thoracic surgical intervention and shows unfavourable outcome in significant number of patients. Pleural fluid and sputum AFB smear and rapid molecular diagnostic test like CBNAAT should be sent in all cases of empyema. Pleural fluid CBNAAT is very sensitive and can clinch the diagnosis in most of the cases with additional advantage of identifying drug resistance to rifampicin. Availability of CBNAAT has the potential to increase the early diagnosis of tubercular empyema with scope for early identification of drug resistant cases. Prompt administration of antitubercular drugs and early drainage of pus will probably result in favourable outcome in these patients.