Primary Hyperparathyroidism (PHP) is a rare event in pregnancy; Maternal complications in PHP patients can be as high as 67%. It can be overlooked easily because of many similar complaints shared by hyperparathyroidism and pregnancy such as nausea and vomiting, gastritis, bone aches, easy fatigability. Hypercalcemic crisis can develop resulting in coma and death. Neonatal effects are tetany and death in about 80% of cases. We report a case, of an antenatal woman at 30 weeks gestation with complains of painful swelling in left lower jaw and below right knee, pain over right hip joint and frequent episodes of gastritis. She was finally diagnosed to have primary hyperparathyroidism and brown tumour due to parathyroid adenoma. The baby was kept in Neonatal Intensive Care Unit (NICU) for three weeks, in view of prematurity with respiratory distress and later developed sepsis with DIC. The patient’s signs and symptoms regressed after parathyroid surgery and the baby was healthy at the time of discharge. This case highlights the progressive deterioration of the patient because of lack of awareness of this disease process and its impact on maternal and foetal morbidity.
Case Report
A 25-year-old G2A1 at 30 weeks gestation presented to Gynaecology OPD with complaint of painful swelling in left lower jaw and below right knee, pain over right hip joint. She also complained of frequent episodes of gastritis during the pregnancy and five months prior to conception. There was no history of any acute pain abdomen, dysuria, constipation, weight loss, anorexia, polyuria and polydipsia, renal stones, fractures, osteoporosis or endocrinopathies. She never consumed drugs like thiazides, diuretics, antacid or lithium. She was on irregular consumption of tablet voveran, calcium and vitamin D3, for past eight months.
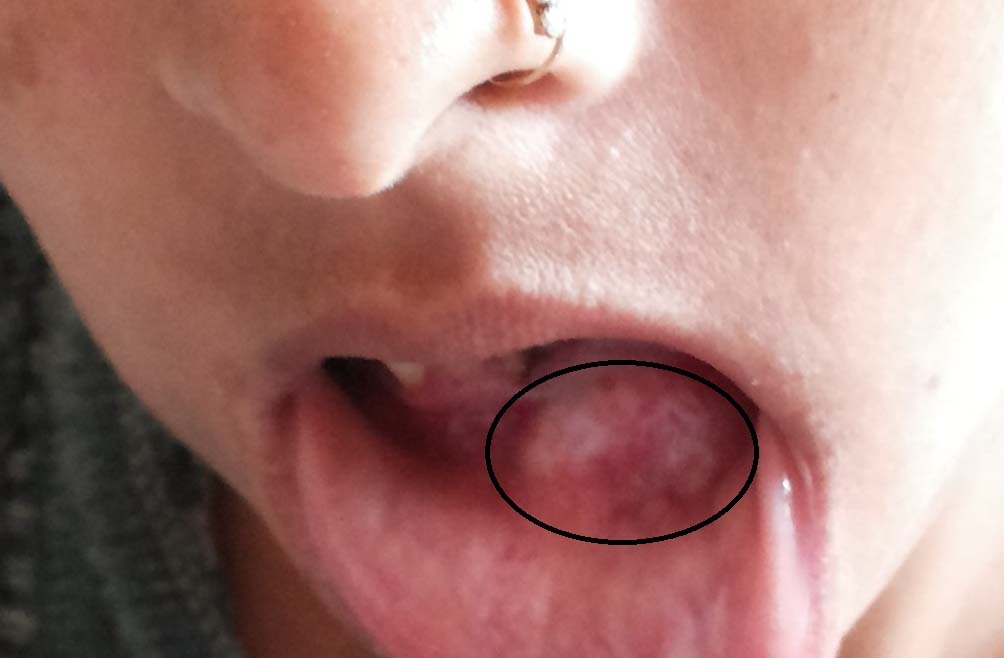
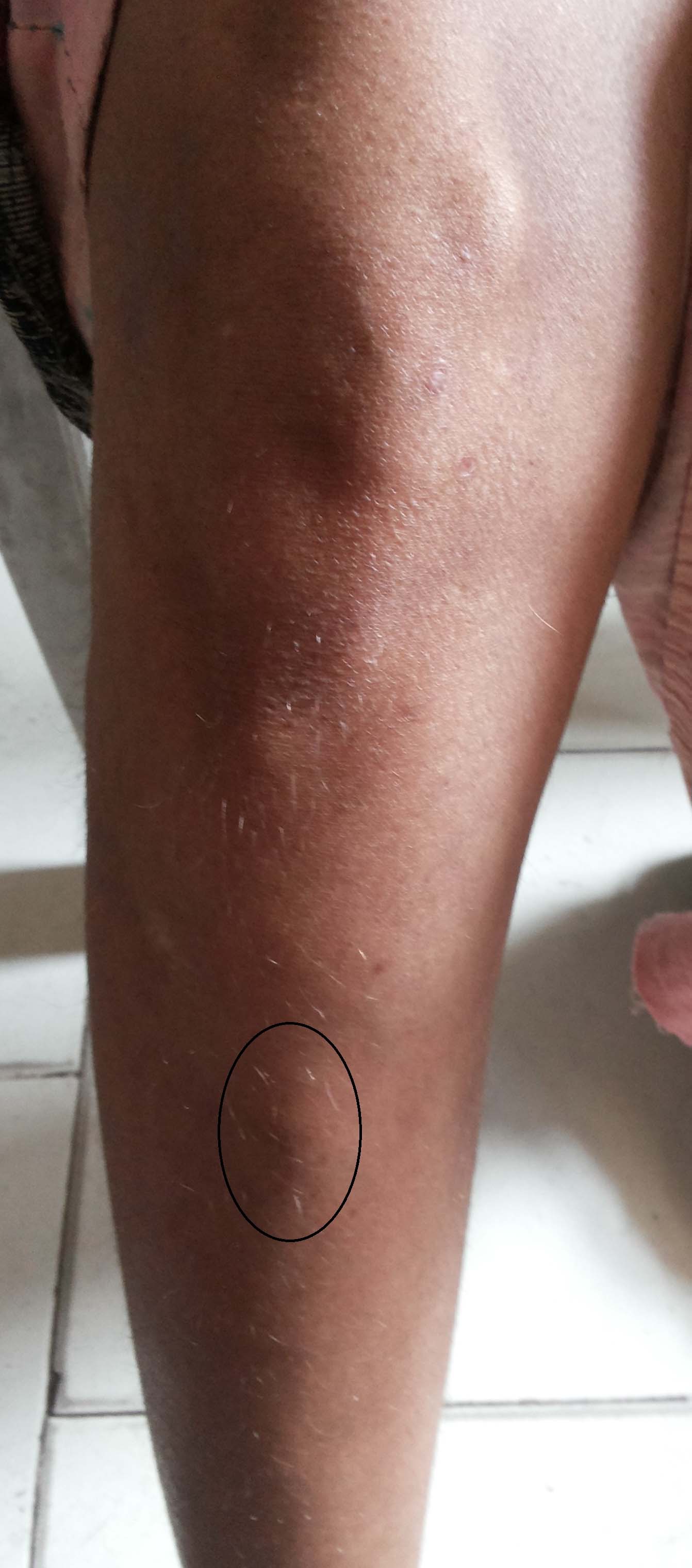
She was thin built but well nourished. An intraoral fixed, non tender, firm-hard, smooth surfaced mass of 2 x 3 cm was palpated over left side of the body of the mandible [Table/Fig-1]. And another swelling of 6 x 5 cm of similar nature was found just below the right knee over the tibia [Table/Fig-2].
Brown’s tumour – mandible.
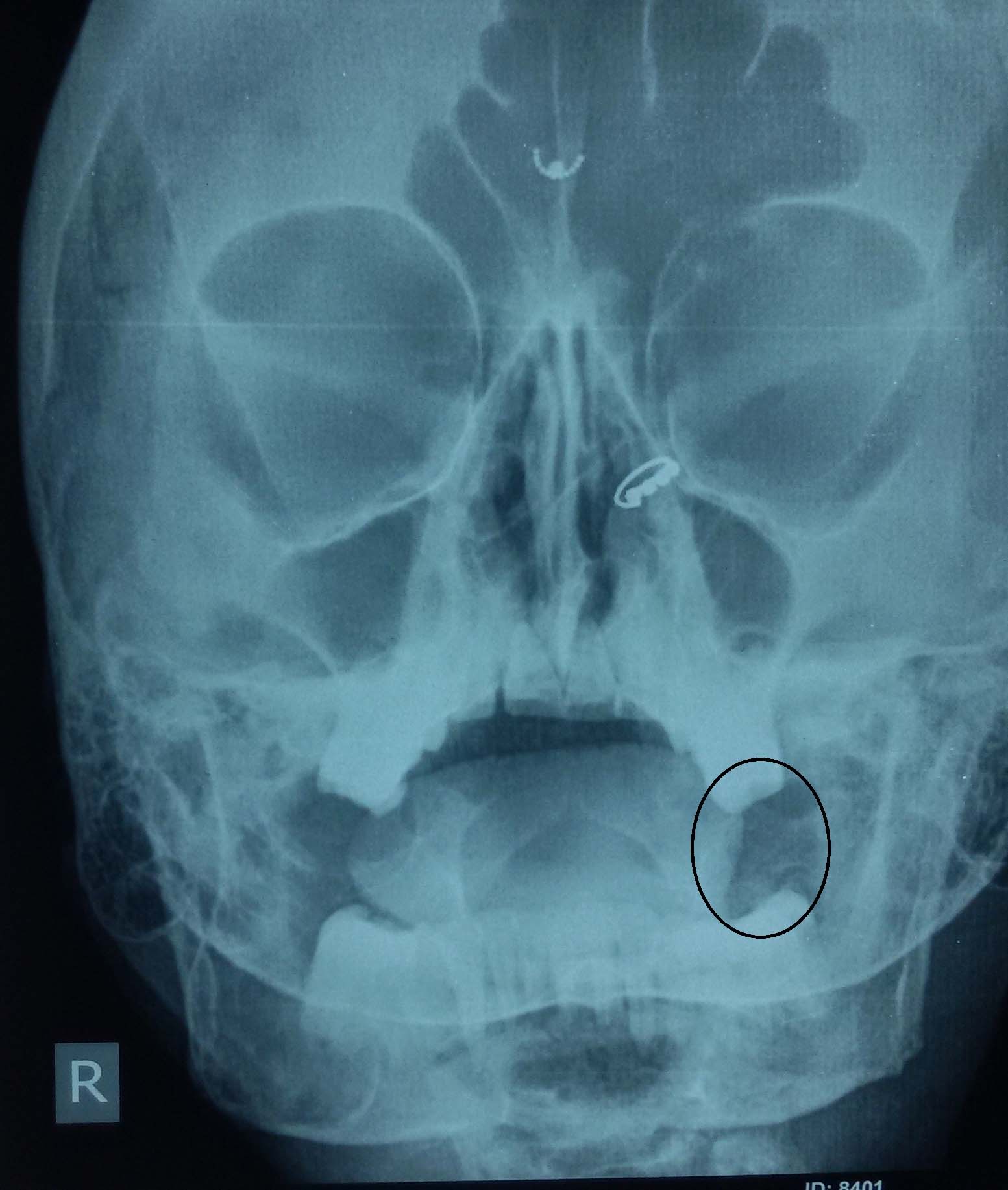
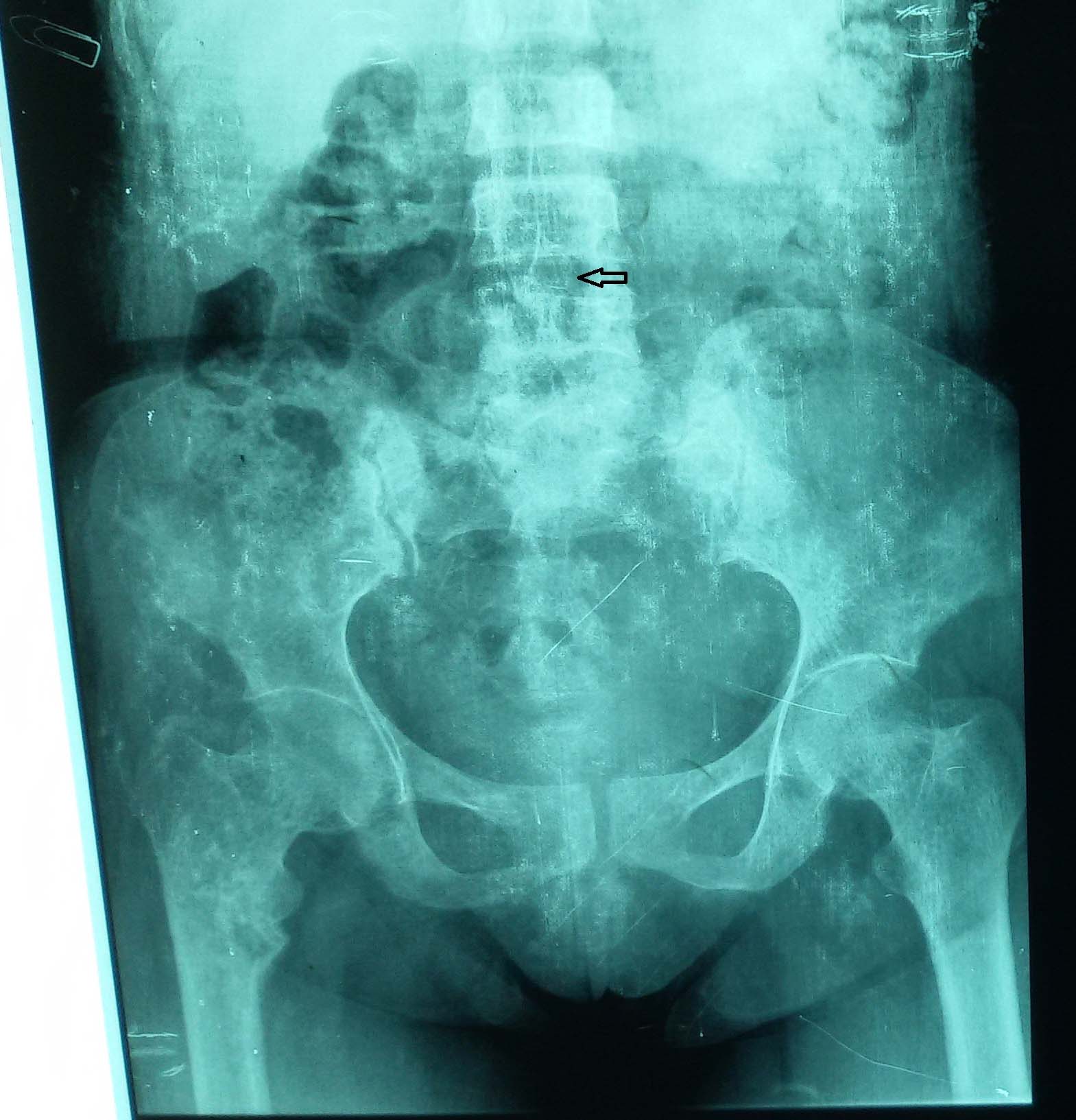
Abdominal examination revealed that the fundal height corresponded to 28 weeks with live foetus in cephalic presentation. The rest of the general and systemic examination was normal. Her baseline investigations were within normal limits; serum calcium 11.1 mg/dl (8-11), albumin 3 g/dl (3.2-5.5), phosphorus 2.4 mg/dl (2.5-5.5) and alkaline phosphate 2240 IU/l (14-147 IU/l). Her Parathormone (PTH) levels were 416.5 pg/ml (16-48). Kidney Function Test (KFT) and Thyroid Function Test (TFT) were normal. ECG showed cardiomegaly but normal QTc interval. Multiple expansile lytic lesions were seen on Peripheral Nervous System (PNS), hip joint and vertebral X-rays [Table/Fig-3,4].
X-ray PNS - well defined lytic lesion on left side mandible.
X-ray spine - multiple osteolytic lesions with thinning of cortex seen on lumbar vertebrae.
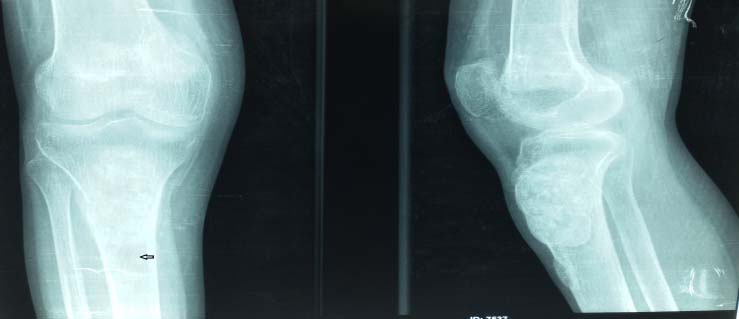
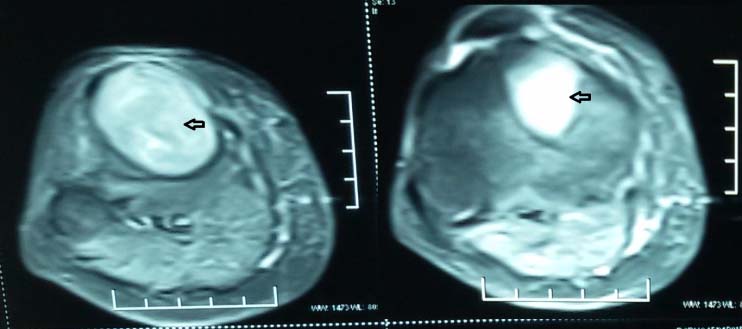
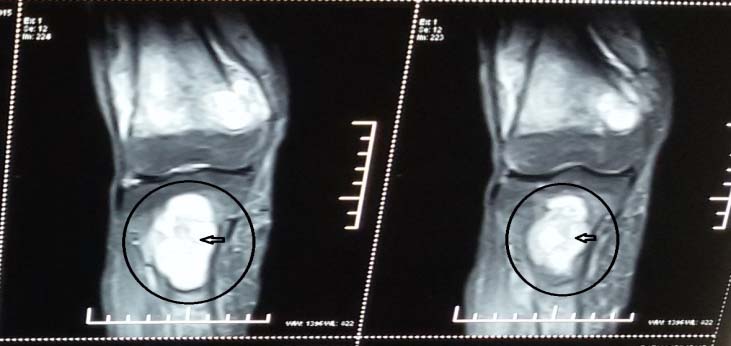
X-ray of right tibial shaft [Table/Fig-5] also showed expansile lytic lesions on metaphysis and epiphysis of 3 cm x 4 cm. USG neck revealed a hypoechoic lesion of 3 cm x 0.8 cm x 1 cm in left parathyroid gland, indicative of parathyroid adenoma. Findings were confirmed on MRI neck and right lower limb [Table/Fig-6,7].
X-ray of femur and tibia – purely lytic lesion.
MRI neck – solid lytic lesion seen as intermediate-low intensity on T1 to T2 weighted images.
MRI of femur and tibia - focal bone resorption depicting solid lytic lesion.
Probable diagnosis of browns tumour with PHP was made. Surgery was deferred due to her advanced gestation. She was managed conservatively on oral hydration, low calcium diet and foetal surveillance. At 32 weeks she presented with preterm rupture of membranes and nonsevere preeclampsia. She delivered a 1.4 kg female child. APGAR score at 5 minutes was 6. The baby was kept in NICU for three weeks in view of prematurity with respiratory distress and later developed sepsis with Disseminated Intravascular Coagulation (DIC). She received antibiotics for 21 days and then discharged.
Patient was re-evaluated in post partum period. Calcium levels increased to 12.6 mg/dl, phosphate levels were 2.4 ng/ml, 24 hour urinary calcium was 3135 mg (normal upto 300 mg). Repeat radiographs of paranasal sinus, hip joint with bilateral femur, tibia and vertebrae, showed lytic lesions with internal septation which were suggestive of brown tumour.
An orthopaedician’s opinion was sought and a cast in the right leg was applied. Elective parathyroidectomy of parathyroid adenoma was done after two weeks postpartum. The histopathological examination showed 3 cm x 2 cm x 1.5 cm encapsulated parathyroid adenoma of weight 8.22g, consisting of chief cells, oxyphil cells with cystic changes and no evidence of malignancy [Table/Fig-8,9].
Histopathology showing parathyroid adenoma (H and E stain 4x). Encapsulated, homogenous lesion composed of chief cells and oxyphil cells in capillary network. No vascular, tissue or adjacent invasion.
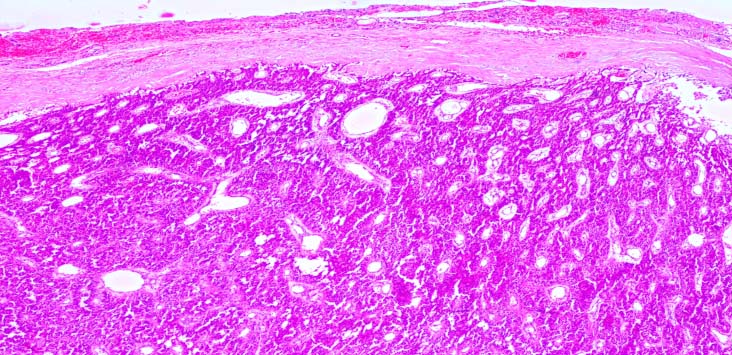
Histopathology showing parathyroid adenoma (H and E stain 4x). Chief cells and oxyphil cells in capillary network are clearly seen.
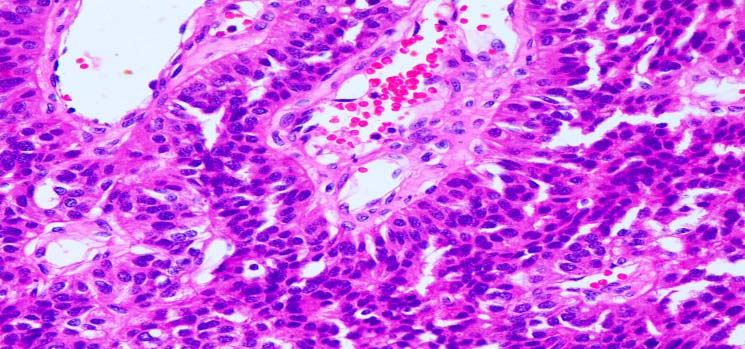
Her post-operative period was uneventful. Patient was reassessed after one month, her jaw and leg swellings had disappeared. Her PTH level decreased to 42 ng/L and subsequent recovery occurred with oral calcium after the transient phase of hypocalcaemia.
Discussion
The common endocrine disorders are diabetes, hypo or hyperthyroidism and PHP among general population [1]. Occurrence of PHP during pregnancy is 8/100,000 population /year [2].
Pathogenesis: The various physiological changes occurr during pregnancy like haemodilution, increased glomerular filtration rate causing hypercalciuria and calcium transport across the placenta to foetus, may mask the presentation of PHP and thus it usually remain undiagnosed during pregnancy. Total serum calcium levels are low and mild hypercalcaemia can only be picked up if correction for lower albumin is done, however ionized calcium levels are similar to the non-pregnant state [2].
There are three major pathological conditions which are associated with PHP: Adenoma, hyperplasia, and carcinoma. Single adenomas account for approximately 89% of all adenomas, hyperplasia 6% and carcinoma in 1-2% of PHP cases [3]. Brown tumours are non-neoplastic lesions resulting from abnormal bone metabolism in hyperparathyroid state that creates a local destructive phenomenon [4,5]. The ribs, femora and pelvis are the most common sites involved [3]. This bony lesion of the HPT is caused by increased circulating levels of parathyroid hormone, which result in increased osteoclastic bone resorption, primarily in cortical bone [6]. Mandible, a cortical bone, is the most commonly affected site [7] and maxillary involvement is less common [8,9].
Clinical manifestations: Hypercalcaemia in pregnancy related to PHP, may be asymptomatic or with symptoms like nausea, vomiting, anorexia, weakness and fatigue. Neuro-psychiatric manifestations, nephrolithiasis, pancreatitis might also be present [10], while pancreatitis is considered an ominous sign of disease severity. Maternal complications related to PHP are 67% and hypercalcemic crisis results in coma and death during postpartum period [11]. Foetal complications are reported in 80% of the cases and 30% of the affected infants may have transient neonatal hypocalcaemic tetany [10,11]. Hella Hultin et al., described that women with parathyroid adenoma are six times more prone for preclampsia. Calcium metabolism and regulatory mechanisms are altered even after successful treatment of parathyroid adenoma, which may be the risk for preclampsia [12]. Schnatz reported, 25% occurrence of preclampsia among women with PHF [13].
Diagnosis and Treatment: The diagnosis is mainly by exclusion and should be considered with parallel findings of elevated total serum calcium level (>9.5 mg/dl) or ionized calcium (<2.5 mg/dl) and increased serum PTH levels [14]. It is necessary to exclude familial hypocalciuric hypercalcaemia, wherein the findings are an inappropriately low/normal 24 urinary calcium excretion in the setting of a high serum calcium level, high/normal PTH. The finding of multiple adenomas should prompt familial screening studies in search for multiple endocrine neoplasia types 1 and 2A, familial hyperparathyroidism and familial hypocalciuric hypercalcaemia.
Ultrasonography of the neck is the investigation of choice during pregnancy for localization of parathyroid adenomas with a sensitivity of 69% and a specificity of 94% [15]. Since the occurrence of hyperparathyroidism during pregnancy is rare, no definite treatment guidelines have been framed, but a female with mild hypercalcaemia (12 mgdL) can be managed with oral hydration, low calcium diet, and strict foetal surveillance [16]. Other measures included in medical management are eucalcaemic diet, oral phosphates 1.5-2.5 gm day and furosemide. Subcutaneous calcitonin can be used safely during pregnancy which acts through direct inhibition of osteoclast action, but has limited value due to tachyphylaxis [17]. Hypercalcemic crisis (calcium level 14 mg/dL) is characterized by development of prerenal failure. Close feto-maternal surveillance is mandatory to minimize significant risks.
Gestation-wise management: Hyperparathyroidism in first trimester should be managed medically [18]. A minimally invasive parathyroidectomy during second trimester (if serum calcium > 11.5 mg/dl) is the therapeutic gold standard management of PHP during pregnancy because the organogenesis is complete and the risk of anaesthesia induced preterm delivery is least. Surgery in 3rd trimester is associated with significant neonatal complications and mortality [19]. If the patient presents in 3rd trimester then benefit and risk has to be explained, surgery should be done if it outweigh the risk. Surgical intervention should be done at any time regardless of the gestational age, if the patient becomes hypercalcemic or symptomatic despite medical management [13]. Our case presented in 3rd trimester and she could be managed conservatively, so parathyroidectomy was planned after delivery.
Conclusion
PHF is a rare disorder presenting during pregnancy; it poses a diagnostic dilemma during pregnancy, because of many similar complaints shared by hyperparathyroidism and pregnancy such as nausea and vomiting, gastritis, bone aches, easy fatigability. However, it is a preventable cause of foetal and maternal morbidity and mortality.
[1]. Felger EA, Kandil E, Primary hyperparathyroidismOtolaryngol Clin North Am 2010 43(2):417-32. [Google Scholar]
[2]. Lopez JM, Fardella CB, Primary hyperparathyroidism: changes on biochemical and hormonal profile related to pregnancyJ Endocrinol Invest 1989 12(2):127-29. [Google Scholar]
[3]. Dinkar AD, Sahai S, Sharma M, Primary hyperparathyroidism presenting as an exophytic mandibular massDentomaxillofac Radiol 2007 36(6):360-63. [Google Scholar]
[4]. Suarez-Cunqueiro MM, Schoen R, Kersten A, Klisch J, Schmelzeisen R, Brown tumour of the mandible as first manifestation of atypical parathyroid adenomaJ Oral Maxillofac Surg 2004 62(8):1024-28. [Google Scholar]
[5]. Leal CT, Lacativa PG, Gomes EM, Nunes RC, Costa FL, Gandelmann IH, Surgical approach and clinical outcome of a deforming brown tumour at the maxilla in a patient with secondary hyperparathyroidism due to chronic renal failureArq Bras Endocrinol Metabol 2006 50(5):963-67. [Google Scholar]
[6]. Daniels JS, Primary hyperparathyroidism presenting as a palatal brown tumourOral Surg Oral Med Oral Pathol Oral Radiol Endod 2004 98(4):409-13. [Google Scholar]
[7]. Emin AH, Suoglu Y, Demir D, Karatay MC, Normocalcaemic hyperparathyroidism presented with mandibular brown tumour: report of a caseAuris Nasus Larynx 2004 31(3):299-304. [Google Scholar]
[8]. Triantafillidou K, Zouloumis L, Karakinaris G, Kalimeras E, Iordanidis F, Brown tumours of the jaws associated with primary or secondary hyperparathyroidism. A clinical study and review of the literatureAm J Otolarygol 2006 27(4):281-86. [Google Scholar]
[9]. Merz MN, Massich DD, Marsh W, Schuller DE, Hyperparathyroidism presenting as brown tumour of the maxillaAm J Otolaryngol 2002 23(3):173-76. [Google Scholar]
[10]. Kort KC, Schiller HJ, Numann PJ, Hyperparathyroidism and pregnancyAm J Surg 1999 177(1):66-68. [Google Scholar]
[11]. Kelly TR, Primary hyperparathyroidism during pregnancySurgery 1991 110(6):1028-33. [Google Scholar]
[12]. Hultin H, Hellman P, Lundgren E, Olovsson M, Ekbom A, Rastad J, Association of parathyroid adenoma and pregnancy with preeclampsiaJ Clin Endocrinol Metab 2009 94(9):3394-99. [Google Scholar]
[13]. Schnatz PF, Thaxton S, Parathyroidectomy in the third trimester of pregnancyObstet Gynecol Surv 2005 60(10):672-82. [Google Scholar]
[14]. Lopez JM, Fardella CB, Primary hyperparathyroidism: changes on biochemical and hormonal profile related to pregnancyJ Endocrinol Invest 1989 12(2):127-29. [Google Scholar]
[15]. Reading CC, Charboneau JW, James EM, High resolution parathyroid sonographyAm J Roentgenol 1982 139:539-46. [Google Scholar]
[16]. Bilezikian JP, Khan AA, Potts JT, Guidelines for the management of asymptomatic primary hyperparathyroidism summary statement from the third international workshopJ Clin Endocrinol Metab 2009 94(2):335-39. [Google Scholar]
[17]. Schnatz PF, Curry SL, Primary hyperparathyroidism in pregnancy evidence based managementObstet Gynecol Surv 2002 57(6):365-76. [Google Scholar]
[18]. Schnatz PF, Curry SL, Primary hyperparathyroidism in pregnancy: evidence-based managementObstet Gynecol Surv 2002 57(6):365-76. [Google Scholar]
[19]. Carella MJ, Gossain VV, Hyperparathyroidism and pregnancy: case report and reviewJ Gen Intern Med 1992 7(4):448-53. [Google Scholar]