Papillary Renal Cell Carcinoma with Tubercular Paraaortic Lymphadenopathy: A Blessing in Disguise
Bhavna Sharma1, Purnima Malhotra2, Minakshi Bhardwaj3
1 Senior Resident, Department of Pathology, PGIMER, Dr. Ram Manohar Lohia Hospital, New Delhi, India.
2 Specialist, Department of Pathology, PGIMER, Dr. Ram Manohar Lohia Hospital, New Delhi, India.
3 Professor and Head, Department of Pathology, PGIMER, Dr. Ram Manohar Lohia Hospital, New Delhi, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Purnima Malhotra, Room No. 323, Pathology Department, 3rd Floor, OPD Block, Dr. Ram Manohar Lohia Hospital, Baba Kharak Singh Marg, New Delhi-110001, India.
E-mail: purnimapaliwal@gmail.com
Incidental Renal Cell Carcinoma (RCC) can be found in a tubercular kidney; however, the vice versa i.e., finding tubercular lymphadenopathy after radical nephrectomy for cancer is exceptionally rare. To the best of our knowledge, only two cases of coexistent renal cell carcinoma and tubercular paraaortic lymphadenopathy have been reported till date. Out of these, only one case was of papillary renal cell carcinoma.
We hereby, report a rare case of papillary renal cell carcinoma with coexisting paraaortic tubercular lymphadenopathy, which was upstaged based on radiology findings. This case highlights the importance of considering the possibility of tuberculosis in cancer-associated lymphadenopathy. This can prevent unnecessary lymphnode dissection and inappropriate clinical upstaging in renal cancer patients.
Granulomatous inflammation, Lymph node dissection, Tuberculosis
Case Report
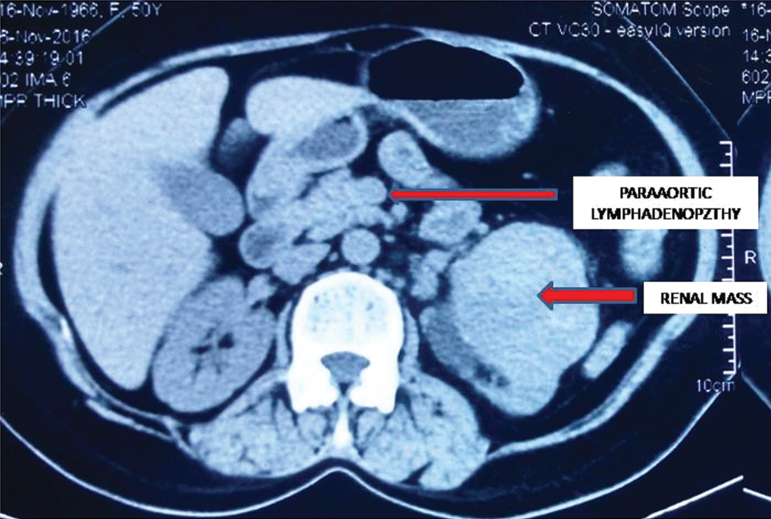
A fifty-year-old female came to urology outpatient department with complaints of episodic painless haematuria along with slowly increasing heaviness in left loin region since six months. Per abdomen examination revealed a left loin mass which was balottable. Contrast Enhanced Computed Tomography (CECT) scan of left kidney revealed a heterogeneously enhancing mass arising from upper pole and interpolar region extending into pelvis and proximal ureter. Multiple heterogeneously enhancing nodes were seen in left paraaortic and aortocaval region [Table/Fig-1]. A provisional clinical diagnosis of renal cell carcinoma metastasizing to regional lymphnodes was made and radical nephrectomy with paraaortic lymphnode dissection was done.
CECT showing renal mass with multiple paraaortic lymphadenopathy;
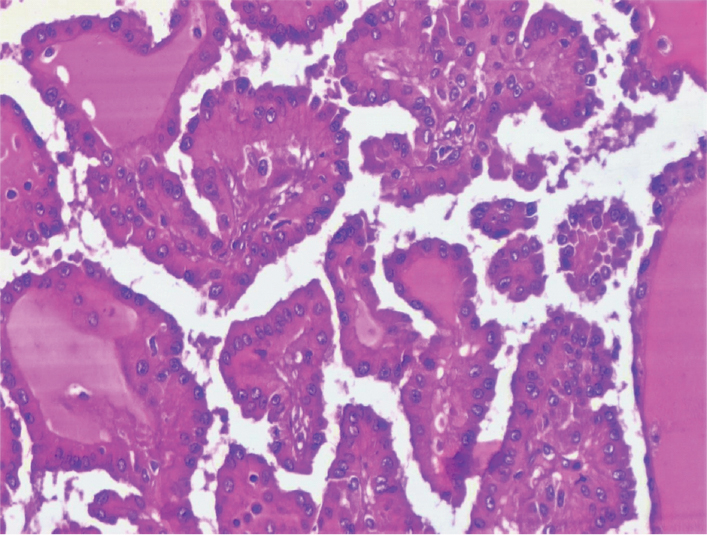
On gross examination, a friable, necrotic tumour adherent to the renal capsule was identified, measuring (6.5 x 6.5 x 6) cm, involving the upper pole and extending to the pelvic region. Histopathology showed papillary renal cell carcinoma Fuhrman nuclear grade three extending into the renal sinus, proximal ureter and the renal vein [Table/Fig-2].
Papillary renal cell carcinoma, Fuhrman nuclear Grade III (H&E, 40X).
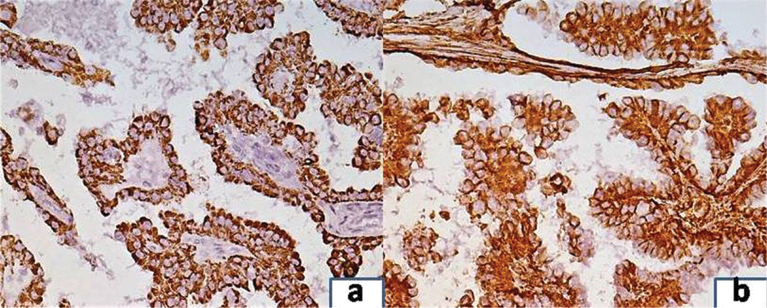
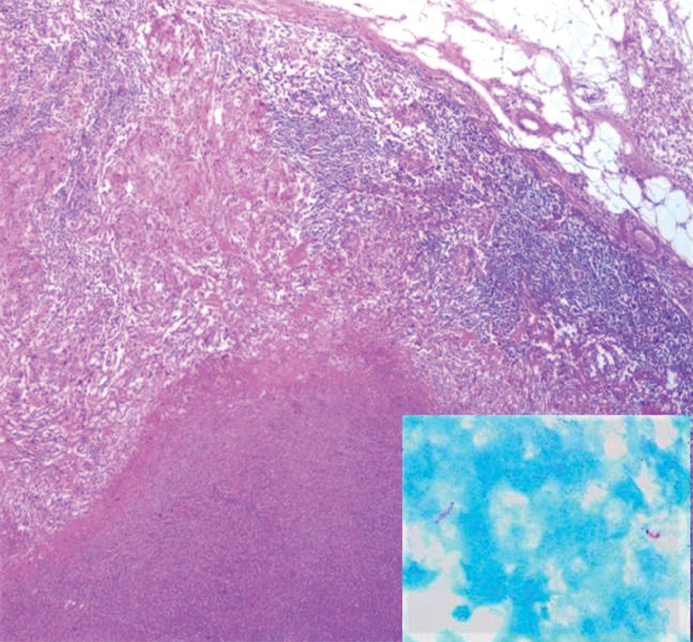
On immunohistochemistry, the tumour cells were positive for both AMACR and vimentin [Table/Fig-3a,b]. The histology of the paraaortic lymph nodes was a complete surprise as all the lymph nodes showed large areas of caseation with multiple epithelioid cell granulomas [Table/Fig-4,5]. No tumour deposits were seen. Ziehl-Neelsen (ZN) stain for acid-fast bacilli was positive ([Table/Fig-5], inset). Lung fields were clear on chest X-Ray examination. There was no past history of tuberculosis and family history of contact with tuberculosis was elicited in retrospect. The patient was referred to Directly Observed Treatment, Short-Course (DOTS) centre and was started on Antitubercular Treatment (ATT). The patient was lost to further follow up.
a) Tumour cells showing positivity for AMACR immunostain (20X); b) Tumour cells showing positivity for vimentin immunostain (20x).
Paraaortic lymphnode showing epithelioid cell granuloma with langhans giant cell (H&E, 40X).
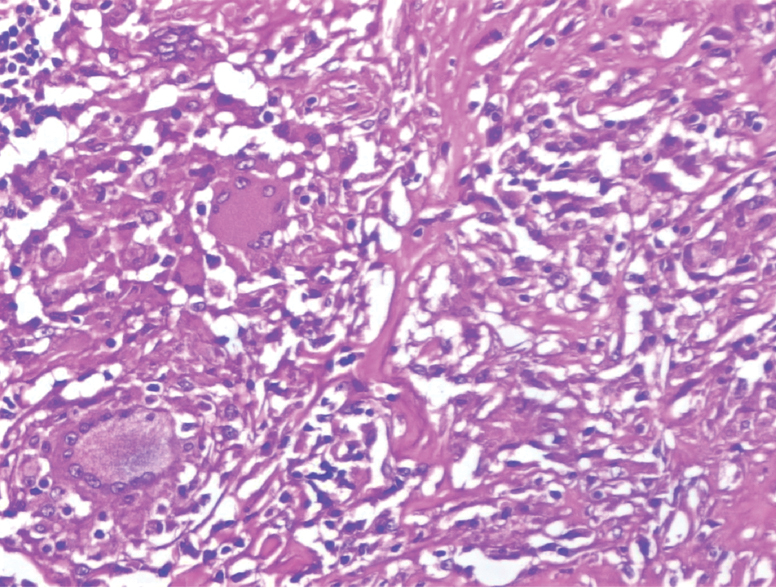
Paraaortic lymphnode showing caseation necrosis (H&E, 20X); Inset. Acid fast bacilli seen in lymphnode (ZN, 100X).
Discussion
The coexistence of tuberculosis and renal cell carcinoma is uncommon, with less than fifty cases reported in literature [1,2]. The coexistence of renal cell carcinoma with tubercular paraaortic lymphadenopathy is very rare. To the best of our knowledge, only two cases of coexistent renal cell carcinoma and tubercular paraaortic lymphadenopathy have been reported till date [3,4]. One of these was a clear cell carcinoma which showed tubercular granulomas within the tumour in the renal parenchyma vis a vis our case wherein granulomas were noted only in paraaortic lymphnodes. Also, our case is unique as Ziehl-Neelsen stain for acid-fast bacilli was positive unlike the other two reported cases where no acid-fast bacilli were demonstrated.
Various theories have been proposed implicating Mycobacterium tuberculosis as an initiator of tumourigenesis. It has been found that Mycobacterium tuberculosis can produce reactive oxygen species and nitric oxide which cause DNA damage [5]. Also, it can induce production of B-cell Lymphoma 2 (Bcl-2) which inhibits apoptosis and leads to tumourigenesis [6].
Granulomatous reaction in cancer draining lymphnodes is a well-known phenomenon. Non-caseating granulomas with unknown aetiology are called sarcoid-like granulomas [7]. These occur due to a T-cell mediated reaction to tumour cells or a soluble tumour antigen, and can be seen in lymph nodes as well as within the tumour [7]. These have been described in Hodgkins lymphoma, Non-Hodgkins lymphoma, various carcinomas, as well as in sarcomas. A wide variety of carcinomas ranging from skin, larynx, cervix, prostate, bladder, ovary, testis, stomach, Gastrointestinal tract (GI tract) and breast have demonstrated granulomatous inflammation [8]. The differential diagnosis of granulomas in cancer draining lymph nodes mainly includes sarcoid-like granulomas, sarcoidosis and tuberculosis. In our case, the diagnosis was straightforward as ZN stain was positive.
Metastasis is found in 20% to 30% of patients with renal cell carcinoma [9]. The 60% of the metastatic renal cell carcinomas present with lymph node involvement, out of which 50% have concurrent distant metastasis [10,11]. Lymph node involvement confers poor prognosis in renal cell carcinoma patients imparting a five year survival ranging from just 5% to 35% [4]. Pantuck et al., reviewed 900 patients of renal cell carcinoma and concluded that positive lymph node status was associated with larger, higher grade and more locally advanced tumours. These tumours were also more likely to demonstrate sarcomatoid features and 3-4 times more likely to have distant metastatic disease [12]. However, the role of lymph node dissection in renal cell carcinoma is controversial. A prospective randomized trial by EORTC concluded that lymph node dissection is not therapeutic in the routine management of renal cell carcinoma. Moreover, lymph node dissection adds significant time, potential morbidity, and requires dissection of and around great vessels [13].
The radiologic findings of tubercular and metastatic lymph nodes show considerable overlap [14]. In our case, the clinical diagnosis of renal cell carcinoma with metastasis to paraaortic lymphnodes was based on radiology findings. The poor prognosis was explained to the patient preoperatively. An unexpected histopathologic diagnosis of tubercular paraaortic lymphadenopathy proved to be a blessing for the patient as her long-term prognosis improved considerably. However, the blessing came in a disguise of unnecessary lymph-node dissection. This experience taught us that extensive lymphadenopathy associated with renal cell carcinoma on imaging does not necessarily signify metastatic disease, especially in our country, where tuberculosis is rampant. Similar findings have been described previously in a single case reported by Pushkar P et al., [3].
Conclusion
Metastatic lymphadenopathy is an independent poor prognostic factor and warrants aggressive surgical resection as an attempt to better disease-free survival. However, the mere presence of extensive lymphadenopathy in renal cell cancer on imaging does not always imply metastasis. In our country, where prevalence of tuberculosis is high, tubercular aetiology should always be considered. Guided aspiration of lymph nodes when feasible and eliciting past or family history of tuberculosis may help circumvent this issue. This can prevent false clinical upstaging and unnecessary lymph node dissection.
[1]. Peyromaure M, Sèbe P, Darwiche F, Claude V, Ravery V, Boccon-Gibod L, Renal tuberculosis and renal adenocarcinoma: a misleading associationProg Urol 2002 12(1):89-91. [Google Scholar]
[2]. Saadi A, Ayed H, Bouzouita A, Kerkeni W, Cherif M, Ben Slama RM, Discovery of renal tuberculosis in a partial nephrectomy specimen done for renal tumourUrol Case Rep 2015 3(3):68-69. [Google Scholar]
[3]. Pushkar P, Agarwal A, Sarin A, Kashyap V, Concurrent RCC with tuberculous paraaortic lymphadenopathy: A pleasant surpriseCan Urol Assoc J 2015 9(3-4):E210-12. [Google Scholar]
[4]. Thottathil M, Verma A, D’souza N, Khan A, Small renal tumour with lymph nodal enlargement: A histopathological surpriseUrol Ann 2016 8(3):391-93. [Google Scholar]
[5]. Shin D-M, Yang C-S, Lee J-Y, Lee SJ, Choi H-H, Lee H-M, Mycobacterium tuberculosis lipoprotein-induced association of TLR2 with protein kinase C xi in lipid rafts contributes to reactive oxygen species-dependent inflammatory signalling in macrophagesCell Microbiol 2008 10:1893-905. [Google Scholar]
[6]. Velmurugan K, Chen B, Miller JL, Azogue S, Gurses S, Hsu T, Mycobacterium tuberculosis nuoG is a virulence gene that inhibits apoptosis of infected host cellsPLoS Pathog 2007 3:e110 [Google Scholar]
[7]. Burhan W, Rowaie ZA, Rajih E, Akhtar M, Sarcoid-like granulomatous reaction in renal cell carcinoma: Report of a case with review of the published reportsAnn Saudi Med 2013 33:614-18. [Google Scholar]
[8]. Brinker H, Sarcoid reactions in malignant tumoursCancer Treat Rev 1986 13:147-56. [Google Scholar]
[9]. Godoy G, O’Malley RL, Taneja SS, Lymph node dissection during the surgical treatment of renal cancer in the modern eraInt Braz J Urol 2008 34:132-42. [Google Scholar]
[10]. Ljungberg B, Words of wisdom. Re: Radical nephrectomy with and without lymph-node dissection: final results of european organization for research and treatment of cancer (EORTC) randomized phase 3 trial 30881Eur Urol 2009 55(6):1486-87. [Google Scholar]
[11]. Canfield SE, Kamat AM, Sánchez-Ortiz RF, Detry M, Swanson DA, Wood CG, Renal cell carcinoma with nodal metastases in the absence of distant metastatic disease (clinical stage TxN1-2M0): the impact of aggressive surgical resection on patient outcomeJ Urol 2006 175(3 Pt 1):864-69. [Google Scholar]
[12]. Pantuck AJ, Zisman A, Dorey F, Chao DH, Han KR, Said J, Renal cell carcinoma with retroperitoneal lymph nodes: role of lymph node dissectionJ Urol 2003 169(6):2076-83. [Google Scholar]
[13]. Jamal JE, Jarrett TW, The current role of lymph node dissection in the management of renal cell carcinomaInt J Surg Oncol 2011 2011:816926 [Google Scholar]
[14]. Yu MG, Atun JM, Tuberculous lymphadenitis mimicking nodal metastasis in follicular variant papillary thyroid carcinomaCase Rep Med 2016 2016:5623104 [Google Scholar]